- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transplantation of Fecal Microbiota Shaped by Diet
Published: Vol 8, Iss 1, Jan 5, 2018 DOI: 10.21769/BioProtoc.2683 Views: 10042
Reviewed by: Neelanjan BoseLucíola da Silva BarcelosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualizing Hypoxia in a Murine Model of Candida albicans Infection Using in vivo Biofluorencence
José Pedro Lopes and Constantin F. Urban
Aug 5, 2019 5103 Views

RNA Extraction from Ears and Draining Lymph Nodes of Mice Infected with Leishmania amazonensis
Emilie Giraud and Evie Melanitou
Jun 5, 2020 5513 Views

TetR Regulated in vivo Repression Technology to Identify Conditional Gene Silencing in Genetically Engineerable Bacteria Using Vibrio cholerae Murine Infections as Model System
Franz G. Zingl [...] Stefan Schild
Oct 5, 2020 3742 Views
Abstract
Alterations in diet and gut microbial ecology underlie the pathogenesis of type 1 diabetes (T1D). In the non-obese diabetic (NOD) mouse, we found high concentrations of bacterial metabolites acetate and butyrate in blood and faeces correlated with protection from disease. We reconstituted germ free (GF) NOD mice with fecal bacteria from protected NOD mice fed with high acetate- and butyrate-yielding diets, to test whether the transferred gut microbiota protect against the development of T1D. GF NOD mice that received a microbiota shaped by high acetate- but not butyrate-yielding diet showed a marked protection against diabetes. This fecal transplantation assay demonstrated the potential for a dietary technology to reshape the gut microbiota that enables specific bacteria to transfer protection against T1D.
Keywords: Gut microbiotaBackground
Changes in the gut microbiota have been observed in a wide variety of illnesses and conditions. A dysbiotic gut microbiota loses homeostatic balance due to changes in the ratios between commensal and pathogenic bacteria. Dysbiosis can be observed when there are large changes in the makeup of the microbiota, with certain species increasing or decreasing in number (Clemente et al., 2012; Rajilic-Stojanovic, 2013). Moreover changes in gut microbiota composition (which may affect metabolites such as SCFAs) associate with many inflammatory diseases (Clemente et al., 2012), including T1D (de Goffau et al., 2013; Endesfelder et al., 2014). For example, patients with, or people who are predisposed to, autoimmune type 1 diabetes typically show a decrease in Firmicutes abundance and an increase in their Bacteroidetes abundance (Giongo et al., 2011). In contrast, various types of inflammatory bowel diseases (IBD), such as ulcerative colitis or Crohn’s disease show the opposite (Frank et al., 2007; Spor et al., 2011). One possibility for treatment of T1D is the use of beneficial bacteria, following their identification and successful trialing. For example, Lactobacillus johnsonii isolated from diabetes resistant rats was able to prevent T1D development in the spontaneous rat model of T1D (Valladares et al., 2010). Likewise, transfer of microbiota from male mice, who are less prone to develop T1D, to female mice reduced their rates of T1D, which correlated with changes in the mouse’s hormone levels (Markle et al., 2013). We used diets that reshape the gut microbiota composition and induced the release of microbial short chain fatty acids (SCFAs). In this study, we have demonstrated that particularly the SCFA acetate and butyrate reduced the onset of T1D in NOD mice (Marino et al., 2017). We wanted to determine if the protective effect was coming directly from the bacteria, or by the produced microbial SCFA acetate or butyrate. We therefore have developed a protocol to harvest the microbiota from diet-fed NOD mice, and transfer it to GF NOD mice, so that we can examine the effects of the altered microbiota and its metabolites on the pathogenesis of T1D.
Materials and Reagents
- 1.7 ml microfuge tubes, autoclaved (Corning, Axygen®, catalog number: MCT-175-C )
- Sterile Petrie dishes 35 x 10 mm (Corning, Falcon®, catalog number: 351008 )
- Sterile 5 ml screw top tubes (Techno Plas, catalog number: P5016SU )
- Gloves
- Donor SPF female NOD mice (NOD mice were derived from Monash Animal Research Platform, Melbourne Australia)
- Recipient germ free female NOD mice, pregnant (GF NOD mice were derived from Germ Free Unit, Walter and Eliza Hall Institute of Medical Research)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S3014 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: NIST2186II )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: NIST200B )
- Ethanol (Merck, catalog number: 818760 )
- 10x phosphate-buffered saline (PBS) (see Recipes)
- Sterile deoxygenated 1x PBS (see Recipes)
- 70% ethanol (see Recipes)
Equipment
- P1000 pipette (Eppendorf, catalog number: 3121000120 )
- Surgical tools
- Fine Iris Scissors, delicate pattern, 11.5 cm straight (Fine science tools, catalog number: 91460-11 )
- Standard pattern forceps, 12 cm straight (Fine Science tools, catalog number: 11000-12 )
- Standard pattern forceps, 12 cm curved (Fine Science tools, catalog number: 11001-12 )
- Gavaging needle, curved 20 G 38 mm (ABLE Scientific, catalog number: ASGN7910 )
- Fine Iris Scissors, delicate pattern, 11.5 cm straight (Fine science tools, catalog number: 91460-11 )
- Laminar hood
- Tissue homogeniser, IKA T10 basic model (IKA, model: T 10 basic), with S10N-5G Dispersing element (IKA, model: S 10 N - 5 G )
- Class II Biological safety cabinet
- Autoclave
- Tabletop centrifuge for 15 ml conical tubes (Eppendorf, model: 5810 )
- Conventional mouse cages
Procedure
- Donor mice preparation
- Co-house donor SPF female NOD mice in the same cage or randomly swap them between cages 3 times per week prior beginning diet treatment.
- Repeat this procedure for 3 weeks prior to starting treatment with the diet.
- Feed 10 week-old donor female NOD mice with diets for 5 weeks and collect fecal and cecal contents at 15 weeks of age. The choice of diet is the variable being examined in this protocol, to see if dietary effects work by altering the microbiota.
- Collect faeces from all the donor mice within the diet group so as to more widely sample the microbiotas present. For every two mice receiving gavages, collect the contents of one mouse’s caecum. Because multiple microbiota collections and donations will occur during the experiment, pooling the faeces samples from all the donor mice in a treatment group is essential to maintain a consistent microbiota transfer.
- Co-house donor SPF female NOD mice in the same cage or randomly swap them between cages 3 times per week prior beginning diet treatment.
- Preparation of mixed cecal and fecal samples
- Faeces collection
- Place individual donor mice that had been fed diets for 5 weeks in a clean empty cage under a laminar hood.
- Collect pellets from the bottom of the cage immediately after they are produced by the mouse, and place in a sterile 1.5 ml microtube containing 500 µl of sterile PBS at room temperature (see Recipes).
- Place individual donor mice that had been fed diets for 5 weeks in a clean empty cage under a laminar hood.
- Cecal content collection
- Sacrifice Donor mouse according to ethical guidelines.
- Before dissection, submerge mouse in 70% ethanol (see Recipes). Dissect the mouse and collect the cecal content within Laminar hood to avoid external contamination. To do this make a longitudinal incision through the skin and peritoneum of the mouse’s abdomen to expose the peritoneal cavity.
- Remove the caecum by cutting it free from the small intestine and colon at the junction of these organs with the caecum (see Figure 1), and place in a sterile Petri dish.
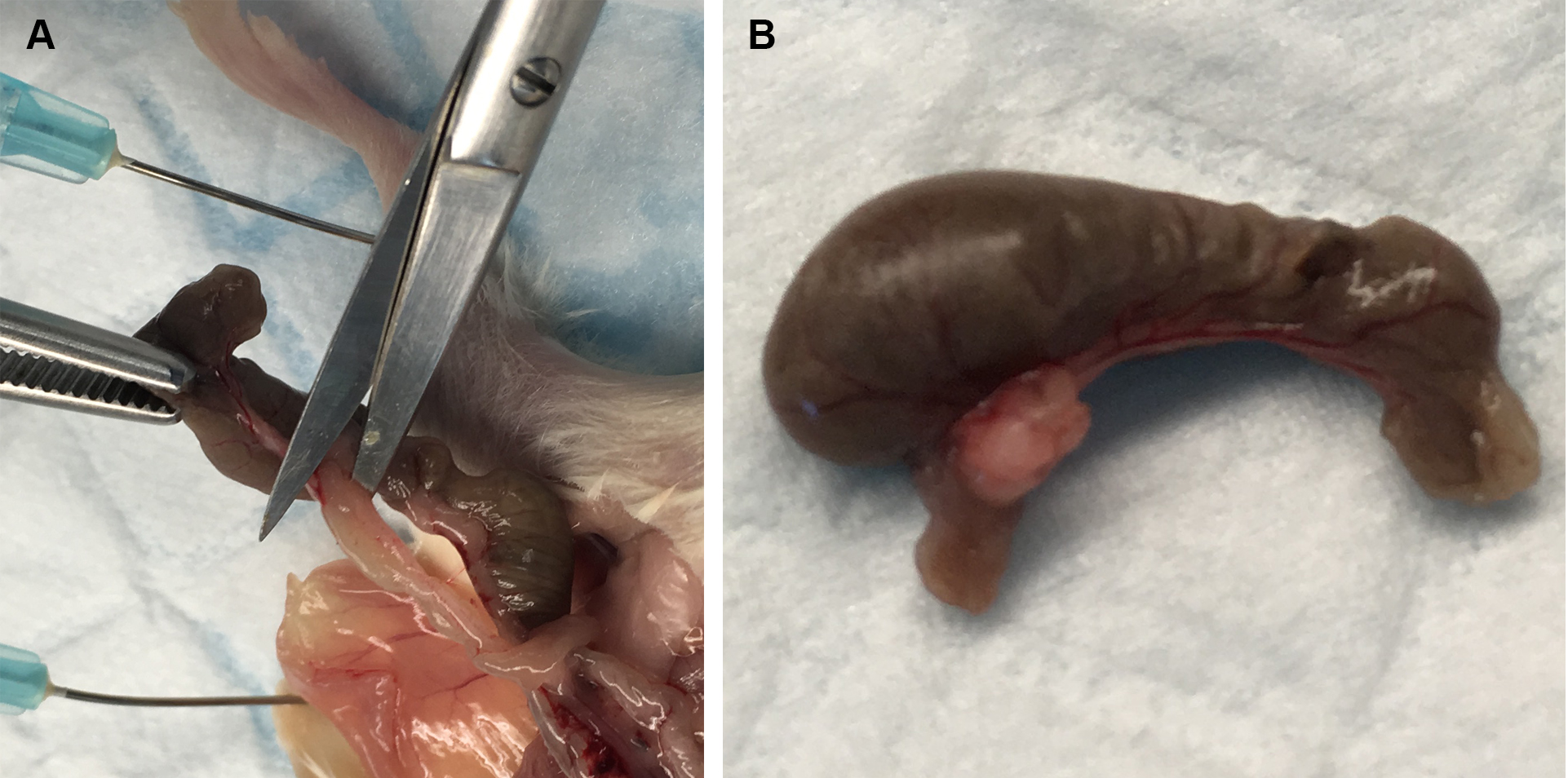
Figure 1. Removal of caecum from mice. A. Cutting of the junction between the caecum and the small and large intestines. B. Caecum after being removed. - Cut off the end of the caecum, and expel the contents into a sterile 5 ml tube containing 1 ml of PBS at room temperature. This is best achieved by using one pair of flat tweezers to hold the caecum in the tube, and a second pair of rounded tweezers to push the contents out into the tube (see Video 1).
Video 1. Removing caecal contents from the caecum
- Sacrifice Donor mouse according to ethical guidelines.
- Faeces collection
- Sample preparation
- Add the faeces in 500 µl of sterile PBS to the 5 ml tube containing the caecal contents in 1 ml sterile PBS for a final volume of ~1.5 ml. Adjust with additional sterile PBS if below this volume.
- Before homogenising samples thoroughly, clean the homogeniser by rinsing it in 70% ethanol (see Recipes) for 1 min twice and in sterile PBS once.
- Resuspend the faeces and caecal contents in the sterile PBS using the homogeniser.
- Spin down the homogenate at 805 x g (RCF) for 10 min at room temperature to pellet and remove solid material, and collect the supernatant which will contain the bacteria.
- Add the faeces in 500 µl of sterile PBS to the 5 ml tube containing the caecal contents in 1 ml sterile PBS for a final volume of ~1.5 ml. Adjust with additional sterile PBS if below this volume.
- Oral gavage
- Diluted homogenates from donors will be administered directly into the stomach of the recipient mice via oral gavage. The diluted cecal content does not generate any risk to the animal’s wellbeing as mice normally eat their own faeces.
- Firstly the animal is firmly restrained to immobilize the head. The mouse is kept in an upright (vertical) position and the gavage needle is gently passed through the side of the mouth, following along the roof of the mouth and is advanced through the oesophagus and toward the stomach. It is important to note that the entire length of the needle must be inserted into the mouse, this will confirm that the needle has entered the oesophagus not the trachea.
- After the needle is passed to the correct length, the microbiome will be injected. Mice don’t need to fast and volumes administered will not exceed 10 ml/kg in mice. If the animal shows any signs of distress after administration begins, the procedure should stop and the needle should be withdrawn immediately. If it appears that material has been injected into the lungs (if the animal shows signs of choking), then the animal should be euthanized. Recipient mice are left to rest for the next 24 h.
- Reconstitute the recipient GF mice with 200 μl of the homogenate supernatant via oral gavage.
- Recipient mice should be observed for no less than 15 min after procedure, and each day for the following two days after the procedure for signs of pain or distress, such as gasping, bleeding or frothing at the mouth. If any of these signs are observed, the mouse should be humanely euthanized.
- After gavage the pregnant GF Recipient Females will then be left alone with ‘Do Not Disturb’ cards for 2 days and will be housed in clean, autoclaved cages, using sterile food, water and bedding. To handle the GF recipient mice use double gloves and perform all work in a laminar hood. The oral gavage needs to be performed by a person experienced in the technique as it is important to be very delicate to avoid disrupting the mother, leading her to kill her pups.
- Inoculate pregnant germ-free NOD female recipient mice with a first oral dose of bacterial mix at the embryonic stage E(13) of pregnancy (measured from observation of plug), and a second oral gavage when pups are 2 weeks-old.
- Pups will be ready to be weaned at 21-24 days of age, and placed on normal chow and normal water. Similar to their mothers, 3-4 weeks old pups will receive two gavages with the donor microbiota, one day apart.
- Once the pups from the recipient mother’s are past their treatment period, they will be monitored for diabetes onset for up to 30 weeks of age. Diabetes assessment will be done by checking the blood glucose levels, with recordings above 12 mmol/L recorded on two consecutive days considered diabetic as previously described (Marino et al., 2009), and compared between groups. Examples of the results can be found in (Marino et al., 2017)
- Diluted homogenates from donors will be administered directly into the stomach of the recipient mice via oral gavage. The diluted cecal content does not generate any risk to the animal’s wellbeing as mice normally eat their own faeces.
Notes
- Due to the nature of the experiment, focusing on transferring the microbiota as accurately as possible from the donor to the recipient it is necessary to make as effort as possible to avoid external contamination. All collection tubes and media should be autoclaved, samples handled in sterile environments and all mice work conducted under the hood.
- Donor mice should be matched to the age and gender of the recipient mice to minimize other variables causing differences in the microbiota beyond diet.
- The co-housing of the donor mice prior to the beginning of the experiment is important to standardize the microbiota of all the mice before the diets are added to alter the microbiota in a specific way.
- Collection of fresh donor material on the day of the donation is highly recommended as storage of the microbiota may lead to altered bacterial populations being present. Storage in the fridge may lead to some species growing, while others remain static, leading to the relative abundances to no longer represent the environment in the gut. Likewise, storage in a freezer may lead to bacterial death, again altering relative populations.
- It is ideal to maintain anaerobic conditions whilst handling the samples, but maintaining this during the experiment is impractical. We took as many practical steps as possible, such as using freshly autoclaved PBS and minimization of the lengths between sample collection and gavage, to try to minimize obligate anaerobes dying off.
- We found performing multiple gavages of the desired microbiota helped ensure that the desired microbiota was efficiently transferred as accurately as possible. Because of this, the mixing of faeces samples from the donor groups is important to keep the transferred microbiota consistent.
- Assessment of the oral gavage after it has been performed could be done by collecting and culturing faeces from the mice post gavage to see if the formerly GF mice now have bacteria present. However, the best way to determine the success of the gavage is by analysis of the recipient microbiota in comparison to the donor samples through next-gen sequencing.
Recipes
- 10x phosphate-buffered saline (PBS)
80 g NaCl
2 g KCl
14.4 g Na2HPO4
2.4 g KH2PO4
Prepare 10x PBS by resuspending all ingredients in distilled water to make up to 1 L
Dilute 10x PBS with distilled water to make 1x PBS
Autoclave 1x PBS to sterilize and deoxygenate PBS fresh for the experiment - 70% ethanol
Dilute 99% analytical grade ethanol in distilled water to 70% concentration
Acknowledgments
This work was supported by Juvenile Diabetes Research Foundation (JDRF, 3-2013-94), Diabetes Australia Research Trust (DART, project grant Y14M1-MARE). We would like to thank H.Y. Goh, Y.Yap (Monash University), C. McKenzie (Germ Free Unit, Walter & Eliza Hall Institute of Medical Research) and Monash University Animal Services (MAS) for their assistance. Competing financial interests: Authors declare no competing financial interests.
References
- Clemente, J. C., Ursell, L. K., Parfrey, L. W. and Knight, R. (2012). The impact of the gut microbiota on human health: an integrative view. Cell 148(6): 1258-1270.
- de Goffau, M. C., Luopajarvi, K., Knip, M., Ilonen, J., Ruohtula, T., Harkonen, T., Orivuori, L., Hakala, S., Welling, G. W., Harmsen, H. J. and Vaarala, O. (2013). Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 62(4): 1238-1244.
- Endesfelder, D., zu Castell, W., Ardissone, A., Davis-Richardson, A. G., Achenbach, P., Hagen, M., Pflueger, M., Gano, K. A., Fagen, J. R., Drew, J. C., Brown, C. T., Kolaczkowski, B., Atkinson, M., Schatz, D., Bonifacio, E., Triplett, E. W. and Ziegler, A. G. (2014). Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes 63(6): 2006-2014.
- Faith, J. J., Ahern, P. P., Ridaura, V. K., Cheng, J. and Gordon, J. I. (2014). Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6(220): 220ra211.
- Frank, D. N., St Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N. and Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104(34): 13780-13785.
- Giongo, A., Gano, K. A., Crabb, D. B., Mukherjee, N., Novelo, L. L., Casella, G., Drew, J. C., Ilonen, J., Knip, M., Hyoty, H., Veijola, R., Simell, T., Simell, O., Neu, J., Wasserfall, C. H., Schatz, D., Atkinson, M. A. and Triplett, E. W. (2011). Toward defining the autoimmune microbiome for type 1 diabetes. ISME J 5(1): 82-91.
- Macia, L., Tan, J., Vieira, A. T., Leach, K., Stanley, D., Luong, S., Maruya, M., Ian McKenzie, C., Hijikata, A., Wong, C., Binge, L., Thorburn, A. N., Chevalier, N., Ang, C., Marino, E., Robert, R., Offermanns, S., Teixeira, M. M., Moore, R. J., Flavell, R. A., Fagarasan, S. and Mackay, C. R. (2015). Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6: 6734.
- Marino, E., Richards, J. L., McLeod, K. H., Stanley, D., Yap, Y. A., Knight, J., McKenzie, C., Kranich, J., Oliveira, A. C., Rossello, F. J., Krishnamurthy, B., Nefzger, C. M., Macia, L., Thorburn, A., Baxter, A. G., Morahan, G., Wong, L. H., Polo, J. M., Moore, R. J., Lockett, T. J., Clarke, J. M., Topping, D. L., Harrison, L. C. and Mackay, C. R. (2017). Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 18(5): 552-562.
- Marino, E., Villanueva, J., Walters, S., Liuwantara, D., Mackay, F. and Grey, S. T. (2009). CD4+CD25+ T-cells control autoimmunity in the absence of B-cells. Diabetes 58(7): 1568-1577.
- Markle, J. G., Frank, D. N., Mortin-Toth, S., Robertson, C. E., Feazel, L. M., Rolle-Kampczyk, U., von Bergen, M., McCoy, K. D., Macpherson, A. J. and Danska, J. S. (2013). Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339(6123): 1084-1088.
- Mayhew, J. W., Onderdonk, A. B. and Gorbach, S. L. (1975). Effects of time and growth media on short-chain fatty acid production by Bacteroides fragilis. Appl Microbiol 29(4): 472-475.
- Rajilic-Stojanovic, M. (2013). Function of the microbiota. Best Pract Res Clin Gastroenterol 27(1): 5-16.
- Round, J. L. and Mazmanian, S. K. (2010). Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107(27): 12204-12209.
- Spor, A., Koren, O. and Ley, R. (2011). Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9(4): 279-290.
- Valladares, R., Sankar, D., Li, N., Williams, E., Lai, K. K., Abdelgeliel, A. S., Gonzalez, C. F., Wasserfall, C. H., Larkin, J., Schatz, D., Atkinson, M. A., Triplett, E. W., Neu, J. and Lorca, G. L. (2010). Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS One 5(5): e10507.
- Wrzosek, L., Miquel, S., Noordine, M. L., Bouet, S., Joncquel Chevalier-Curt, M., Robert, V., Philippe, C., Bridonneau, C., Cherbuy, C., Robbe-Masselot, C., Langella, P. and Thomas, M. (2013). Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11: 61.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
McLeod, K. H., Mason, L. and Mariño, E. (2018). Transplantation of Fecal Microbiota Shaped by Diet. Bio-protocol 8(1): e2683. DOI: 10.21769/BioProtoc.2683.
Category
Microbiology > Microbe-host interactions > In vivo model > Mammal
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










