- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mutant Huntingtin Secretion in Neuro2A Cells and Rat Primary Cortical Neurons
Published: Vol 8, Iss 1, Jan 5, 2018 DOI: 10.21769/BioProtoc.2675 Views: 8562
Reviewed by: Oneil G. BhalalaSalome Calado BotelhoKae-Jiun Chang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurement of CD74 N-terminal Fragment Accumulation in Cells Treated with SPPL2a Inhibitor
Rubén Martínez-Barricarte [...] Jean-Laurent Casanova
Jun 5, 2019 6979 Views

Capillary Nano-immunoassay for Quantification of Proteins from CD138-purified Myeloma Cells
Irena Misiewicz-Krzeminska [...] Norma C. Gutiérrez
Jun 20, 2019 6849 Views

Far-western Blotting Detection of the Binding of Insulin Receptor Substrate to the Insulin Receptor
Jinghua Peng [...] Ling He
Feb 20, 2023 2483 Views
Abstract
Quantitative analysis of proteins secreted from the cells poses a challenge due to their low abundance and the interfering presence of a large amount of bovine serum albumin (BSA) in the cell culture media. We established assays for detection of mutant huntingtin (mHtt) secreted from Neuro2A cell line stably expressing mHtt and rat primary cortical neurons by Western blotting. Our protocol is based on reducing the amounts of BSA in the media while maintaining cell viability and secretory potential, and concentrating the media prior to analysis by means of ultrafiltration.
Keywords: Protein secretionBackground
A number of proteins are secreted from the cell into the extracellular environment via various secretory pathways. These pathways include the conventional secretory pathway following ER-Golgi-plasma membrane route (Lee et al., 2004) and multiple unconventional pathways, such as lysosomal exocytosis, translocation across the plasma membrane and exosome and ectosome release (Zhang and Schekman, 2013). To study these pathways, it is often necessary to analyze proteins secreted from cultured cells into the media. Proteins may be secreted either in free form or in association with vesicular membrane structures such as ectosomes and exosomes (Zhang and Schekman, 2013). Whether these proteins are membrane-associated or not determines the type of approach used for their analysis. To isolate membrane-associated proteins, media is usually subjected to differential centrifugation procedure upon which the protein-containing membranes are sedimented, concentrated and purified from the constituents of the media, which allows for unhampered protein analysis (Momen-Heravi et al., 2013). However, the analysis of free-form proteins is a more challenging task (Chevallet et al., 2007). Two major obstacles are 1) the presence of substantial amounts of bovine serum albumin (BSA) in the serum or other supplements added to most cell culture media, which may mask the protein of interest; and 2) low abundance in the media of at least some of the secreted proteins, including mHtt. This protocol has been developed in order to study secreted mHtt and may be useful for analyzing other low-abundance, free-form proteins in the media.
Protocol optimization
To tackle the issue of excessive BSA, we first monitored cell viability in various media with partial or total reduction of BSA. In the case of Neuro2A cells stably expressing N-terminal 571 amino acids of mHtt with 72 glutamines (Neuro2A-mHtt cells), the optimal medium was Opti-MEM, which not only preserved viability of the cells, but also enhanced the secretion of mutant huntingtin (mHtt) as compared to other media, such as DMEM without supplemented fetal bovine serum (FBS) and HBSS (Figure 1). Moreover, we observed that applying conditioned Opti-MEM (Opti-MEM pre-incubated with naïve Neuro2A cells which do not express mHtt) dramatically enhanced the secretion of mHtt from Neuro2A-mHtt cells, possibly due to enrichment of the media with factors influencing secretion (Figure 1). However, primary cortical neurons were not viable in the presence of Opti-MEM, whereas they survived well in Neurobasal medium, regardless of the addition of BSA-containing B27 supplement (Table 1). Since high amounts of B27 supplement lead to the appearance of BSA accumulates in the region of the membrane where mHtt normally migrates (even when BSA was partially removed by immunoprecipitation) (Figure 2), we opted for Neurobasal media with reduced amount of B27 (0.2%).
To address the issue of low abundance of mHtt in the media, we compared several approaches to concentrate the media and thus enrich mHtt. While TCA precipitation of proteins and immunoprecipitation of mHtt from the media did not give satisfactory results, ultrafiltration of the media proved to be the method of choice for both Neuro2A cells and neurons. We were then able to detect mHtt by immunoblotting (Figure 1).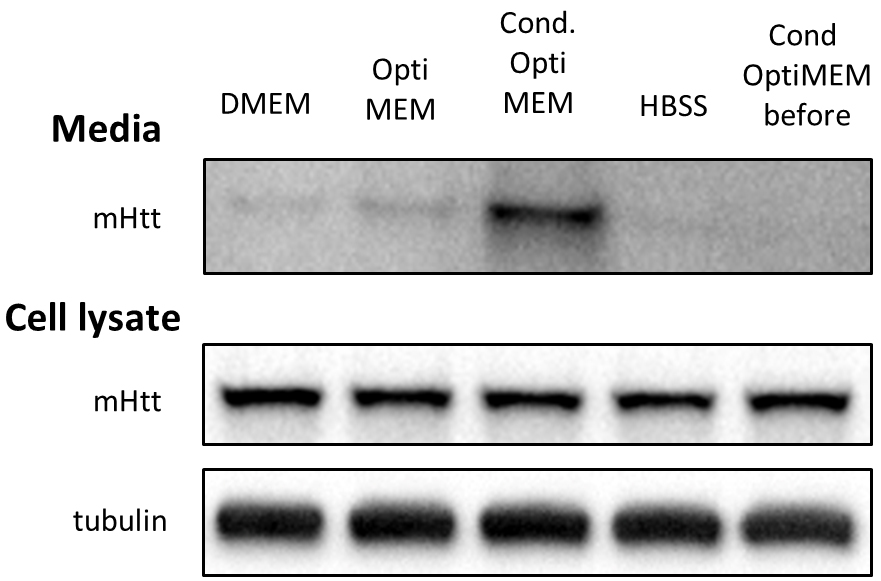
Figure 1. Optimizing conditions for detection of mHtt secreted from Neuro2A-mHtt cells. 800,000 mHtt-Neuro2A cells were incubated for 4 h in the following media: DMEM, Opti-MEM, conditioned Opti-MEM and HBSS. Cells were lysed, and media were collected, concentrated and analyzed by SDS-PAGE/immunoblotting using anti-Htt antibody. Tubulin was used as a loading control for the cell lysates. The fifth lane represents concentrated conditioned media before being applied on mHtt-expressing Neuro2A.
Table 1. Cells from Figure 2 were visually monitored for viability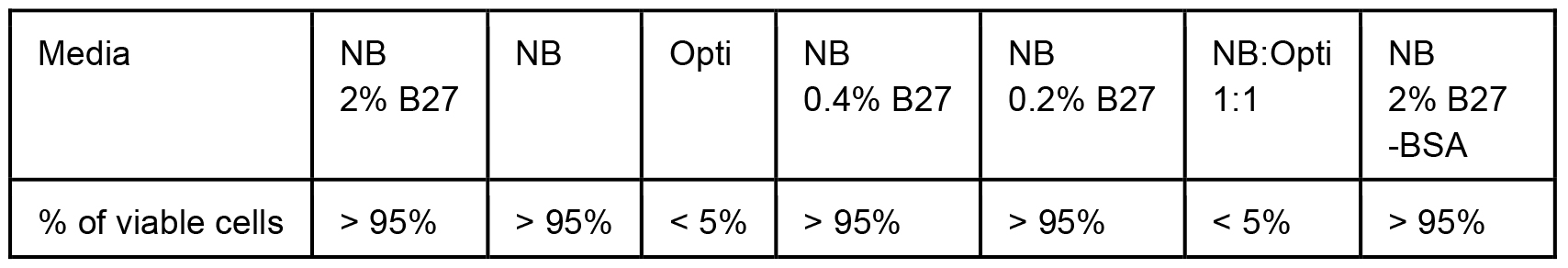
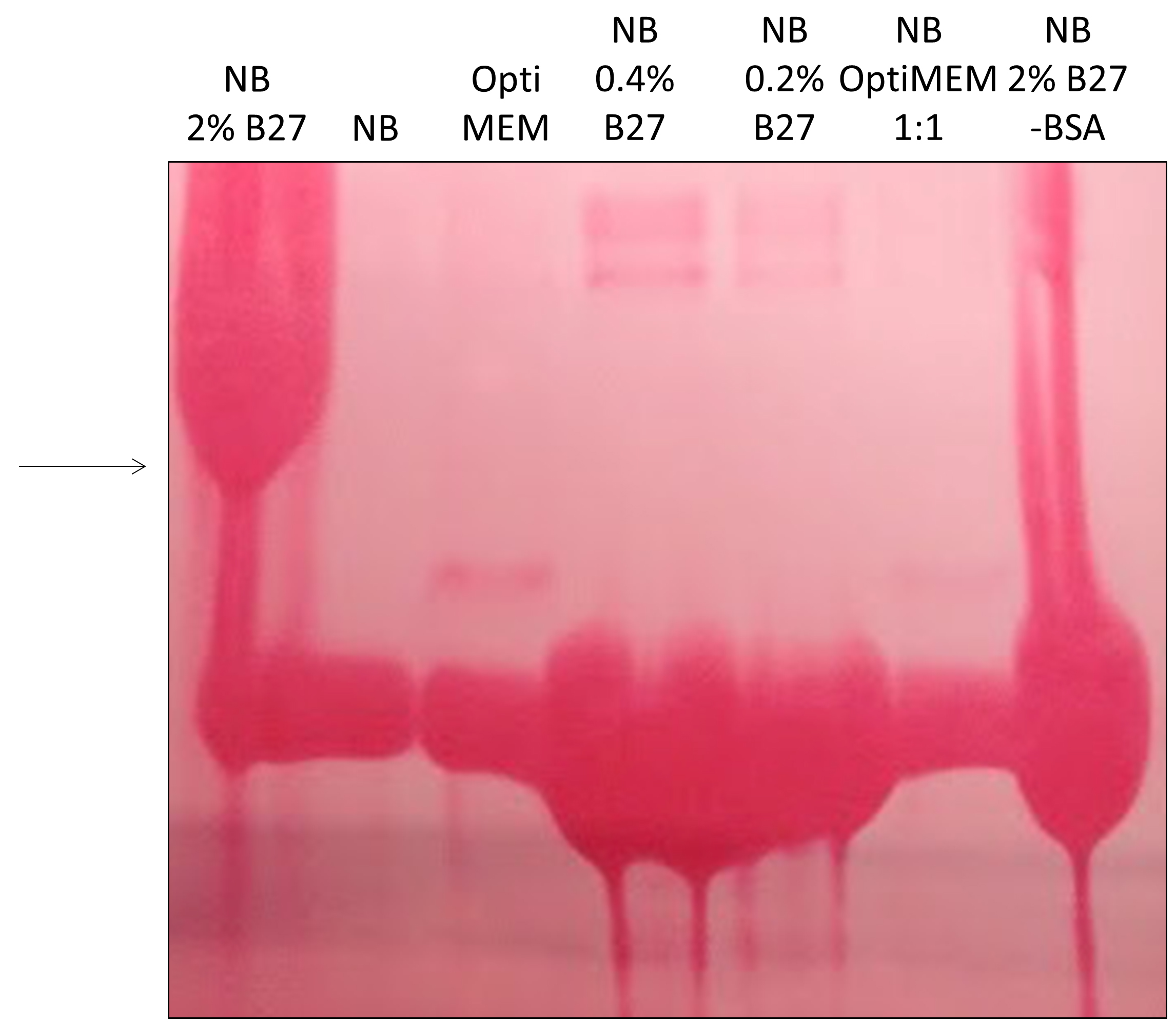
Figure 2. Optimizing conditions for detection of mHtt in primary cortical neurons. 700,000 cells were incubated overnight in Neurobasal media with 2% B27 supplement (NB 2% B27), plain Neurobasal media (NB), Opti-MEM, Neurobasal media with 0.4 or 0.2% B27, a mix of Opti-MEM and Neurobasal media at 1:1, or Neurobasal media with 2% B27 where BSA was partially depleted by immunoprecipitation using anti-BSA agarose beads (NB 2% B27 -BSA). The media were collected, concentrated and analyzed by SDS-PAGE/Western blotting. Membrane was stained by Ponceau S to reveal proteins. Large accumulations of proteins (BSA) in the region where mHtt normally migrates (arrow) revealed that the conditions for lanes 1 and 7 were not optimal for mHtt-immunodetection.
Materials and Reagents
- Pipette tips (Genesee Scientific, Olympus Plastics, catalog numbers: 24-412 , 24-430 , 24-401 )
- 100 mm dishes (Corning, catalog number: 430167 )
- 60 mm dishes (Corning, catalog number: 430166 )
- 12-well plates (Corning, catalog number: 3513 )
- 6-well plates (Corning, catalog number: 3516 )
- 1.5 ml centrifugation tubes (Fisher Scientific, Fisherbrand, catalog number: 05-408-129 )
- Amicon Ultra-0.5 centrifugal filter units, NMWL 10 (Millipore Sigma, catalog number: UFC501096 )
- 15 ml conical tubes (DOT Scientific, catalog number: 229411 )
- 50 ml conical tubes (DOT Scientific, catalog number: 229421 )
- Cell strainer (70 µm, Corning, Falcon®, catalog number: 352350 )
- NuPAGE® Novex® 3-8% Tris-Acetate Gels, 1.0 mm, 15-well (Thermo Fisher Scientific, InvitrogenTM, catalog number: EA03755BOX )
- Neuro2A cells (ATCC, catalog number: CCL-131 )
- Neuro2A-mHtt cells (stably expressing fragment of mHtt from amino acid 1-571 containing 72 glutamines) (Trajkovic et al., 2017)
- mHtt-Flag lentivirus (lentivirus encoding for a fragment of mHtt from amino acid 1-571 containing 72 glutamines with a C-terminal Flag tag)
- Sprague Dawley embryos E18, both sexes (Charles River Laboratories)
- Poly-D-lysine (Sigma-Aldrich, catalog number: P1149 )
- Boric acid (Sigma-Aldrich, catalog number: B7660 )
- Borax (Sigma-Aldrich, catalog number: B9876 )
- Sodium hydroxide (NaOH) (Avantor Performance Materials, catalog number: 5000-02 )
- DMEM (4.5 g/L Glucose, L-Glutamine, Sodium Pyruvate; Thermo Fisher Scientific, InvitrogenTM, catalog number: 11995073 )
- Opti-MEM (Thermo Fisher Scientific, InvitrogenTM, catalog number: 31985088 )
- Fetal bovine serum, certified, US origin (Thermo Fisher Scientific, InvitrogenTM, catalog number: 16000044 )
- 4x Laemmli sample buffer (Bio-Rad Laboratories, catalog number: 1610747 )
- 2x Laemmli sample buffer (Bio-Rad Laboratories, catalog number: 1610737XTU )
- 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148 )
- Milk (Nestle Carnation instant nonfat dry milk)
- TBST (20x, with 2% Tween-20, pH 7.4) (Boston Bio Products, catalog number: IBB-180X )
- Anti-huntingtin antibody MAB5490 (Millipore Sigma, catalog number: MAB5490 )
- Peroxidase-AffiniPure Goat Anti-mouse IgG (H+L) (min X Hu, Bov, Hrs, Rb, Sw Sr Prot) (Jackson ImmunoResearch, catalog number: 115-035-146 )
- SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, catalog number: 34096 )
- 10x HBSS (Thermo Fisher Scientific, InvitrogenTM, catalog number: 14185052 )
- HEPES (Sigma-Aldrich, catalog number: H4034 )
- 100 mM sodium pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11360070 )
- D-glucose (Sigma-Aldrich, catalog number: G7021 )
- Penicillin-streptomycin, 10,000 U/ml (Thermo Fisher Scientific, InvitrogenTM, catalog number: 15140122 )
- Trypsin 2.5% (Thermo Fisher Scientific, InvitrogenTM, catalog number: 15090046 )
- Neurobasal media (Thermo Fisher Scientific, InvitrogenTM, catalog number: 21103049 )
- B27 supplement (50x) (Thermo Fisher Scientific, InvitrogenTM, catalog number: 17504044 )
- L-glutamine, 200 mM (Thermo Fisher Scientific, InvitrogenTM, catalog number: 25030081 )
- Ponceau S solution (Sigma-Aldrich, catalog number: P7170-1L )
- HIV-1 p24 Antigen ELISA (ZeptoMetrix, catalog number: 0 801111 )
- PierceTM LDH Cytotoxicity Assay Kit (Thermo Fisher Scientific, catalog number: 88953 )
- 0.1 M borate buffer (see Recipes)
- DMEM/10% heat-inactivated FBS (see Recipes)
- 0.3 M HEPES (see Recipes)
- HBSS (see Recipes)
- Serum media (see Recipes)
- Full neuronal media (see Recipes)
- Neuronal secretion media (see Recipes)
Equipment
- Automatic pipettes (Gilson, model: Pipetman® L, catalog number: F167370 )
- FormaTM Steri-CycleTM CO2 Incubator, 37 °C (Thermo Fisher Scientific, model: FormaTM Steri-CycleTM )
- S-500 orbital shaker (VWR, model: S-500 , catalog number: 14005-830)
- Eppendorf® refrigerated centrifuge (Eppendorf, model: 5417 R )
- Allegra X-30 centrifuge (Beckman Coulter, model: Allegra® X-30 )
- Bio-Rad Trans-Blot® TurboTM Transfer System (Bio-Rad Laboratories, model: Trans-Blot® TurboTM Transfer System )
- ChemiDocTM XRS+ System (Bio-Rad Laboratories)
Software
- ImageJ software
- Image LabTM Software for ChemiDocTM XRS+ System (Bio-Rad Laboratories)
Procedure
- Detection by immunoblotting of mutant huntingtin secreted from Neuro2A cells (Figure 3)
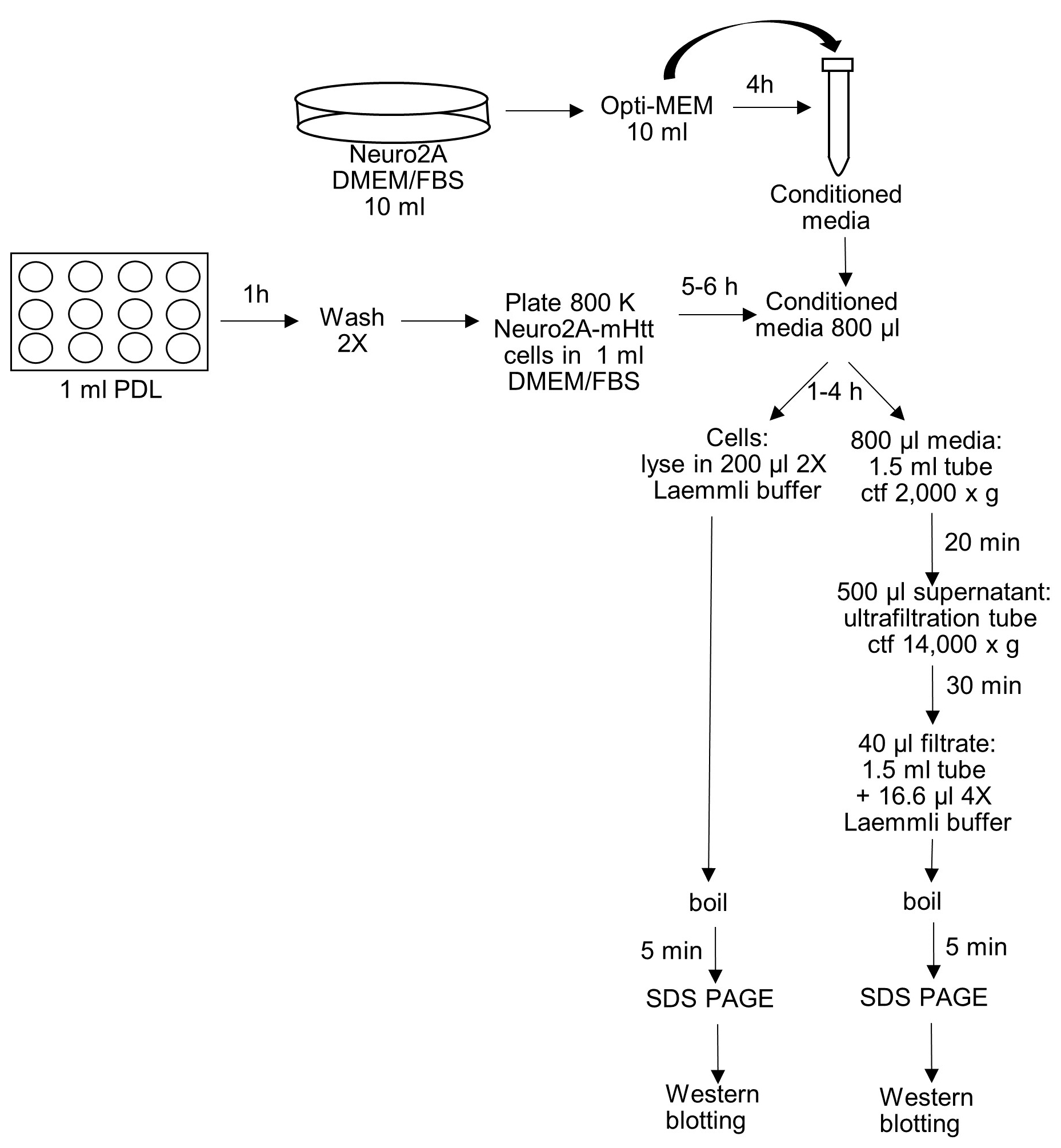
Figure 3. Schematic representation of the protein secretion assay- Coat each well of a 12-well plate with 1 ml poly-D-lysine (50 µg/ml in 0.1 M borate buffer [see Recipes]) for one hour at room temperature (RT); wash the wells two times with 1 ml autoclaved Milli Q water (RT).
- Plate 800,000 of Neuro2A-mHtt cells onto each well and add DMEM/10% heat-inactivated FBS (37 °C; see Recipes) to a total volume of 1 ml.
- Place the cells in an incubator with 5% CO2 at 37 °C and incubate them for 5-6 h.
- During this time, prepare conditioned media using a 100 mm dish containing a confluent layer of naïve Neuro2A cells (Neuro2A not expressing mHtt) grown in 10 ml DMEM/10% heat-inactivated FBS. Replace the media with 10 ml of Opti-MEM and incubate the cells for 4 h in the incubator with 5% CO2 at 37 °C. Collect the resulting conditioned Opti-MEM and use immediately in Step A5.
- Replace the media from Neuro2A-mHtt cells from Step A3 with 800 μl of freshly conditioned Opti-MEM from Step A4 and incubate the cells for additional 1-4 h. Before analysis of mHtt secretion, transfer the media from each well to individual 1.5 ml centrifugation tubes and place the tubes on ice. Lyse the cells on ice in 200 µl of 2x Laemmli buffer with 5% 2-mercaptoethanol, transfer the lysates to 1.5 ml centrifugation tubes and store them on ice while processing the media.
- Centrifuge the media at 2,000 x g for 20 min to remove cell debris. Transfer 500 µl of the cleared supernatant to a centrifugal filter unit and centrifuge for 30 min at 14,000 x g. Transfer the resulting concentrated media (~40 μl) to a clean centrifugation tube and add ⅓ of its volume (13.3 μl) of 4x Laemmli buffer with 10% 2-mercaptoethanol. Handle the media and perform all centrifugation steps at 4 °C.
- Boil cell lysates and the supernatants for 5 min and then place them on ice.
- Load 15 μl per well of the concentrated media in Laemmli buffer and 8 μl per well of cell lysates on 3-8% Tris-Acetate gels. Separate proteins by SDS-PAGE. Transfer the proteins from the gels onto nitrocellulose membranes using Bio-Rad Trans-Blot® TurboTM Transfer System.
- Incubate the membranes for 1 h in 5% milk dissolved in TBS-Tween (RT), then overnight in anti-Htt antibody dissolved at 1:1,000 in 5% BSA/TBS-Tween (4 °C). Wash the membrane 3 x 5 min in TBS-Tween and incubate for further 2 h in the secondary antibody diluted at 1:3,000 in TBS-Tween (RT). Wash the membrane 3 x 5 min in TBS-Tween (RT). Perform all washes and incubations with milk and antibodies on a shaker, level 2. Expose the membrane to West Femto Substrate and reveal mHtt using ChemiDoc imaging system.
- A representative example of the data obtained using this protocol is illustrated in Figure 1.
- Coat each well of a 12-well plate with 1 ml poly-D-lysine (50 µg/ml in 0.1 M borate buffer [see Recipes]) for one hour at room temperature (RT); wash the wells two times with 1 ml autoclaved Milli Q water (RT).
- Detection by Western blotting of mutant huntingtin secreted from primary cortical neurons
Preparation of primary cortical neurons- Coat each well of 6-well plates with 2 ml of poly-D-lysine (50 µg/ml in 0.05 M borate buffer [see Recipes]) overnight (RT). Wash each well with 2 ml autoclaved Milli Q water 1 x 5 min, 1 x 1 h, 1 x overnight, then 2 x 1 h on the next day (RT). Do not let the plates dry at any time. Fill each well with 1 ml serum media (see Recipes).
- Extract brains from rat embryos at E18 and dissect them in HBSS (see Recipes). Remove meninges and place cortices in 5 ml of HBSS in 60 mm dishes on ice.
- Chop the tissue into small pieces in HBSS in the tissue culture hood. Transfer the chopped tissue together with HBSS to a 15 ml conical tube. This and the following steps should be performed at RT, unless indicated otherwise.
- Let the tissue fall to the bottom, aspirate most of the supernatant, add 10 ml HBSS and repeat this step 3-4 times. Last time, leave 4.5 ml of the supernatant, add 0.5 ml 2.5% trypsin and mix by flipping the tube. Incubate for 15 min at 37 °C.
- Add 5 ml HBSS and gently mix by flipping the tube. Remove all but 2 ml of the supernatant using a pipette.
- Dissociate the tissue by pipetting up and down 10 x using P1000 pipette. Filter the obtained cell suspension using a cell strainer and collect the filtrate into a 50 ml conical tube. Rinse the cell strainer with 4 ml HBSS and collect the filtrate into the same tube.
- Transfer the filtrate into a 15 ml conical tube and spin at 500 x g for 5 min at RT. Aspirate the supernatant.
- The pellet will be mostly white, with some red cells at the bottom. To ensure the purity of the culture, dislodge only the white part of the pellet by tapping the tube (red cells are more compact and will remain attached to the bottom of the tube) and resuspend the cells in 4 ml/brain of serum media within the same tube (see Recipes). Transfer the cell suspension into a new conical tube.
- Plate 700,000 cells per well in poly-D-lysine-coated 6-well plates with a total of 2 ml serum media.
- After 2 h, replace the serum media with 2 ml of full neuronal media (see Recipes).
Detection of mutant huntingtin by Western blotting- At DIV (days in vitro) 3, transduce primary cortical neurons with mHtt-Flag lentivirus at 5 MOI (virus equivalent of 700 ng p24; 1 ng p24 contains 5,000 infectious particles).
- After 24 h, replace the media with full neuronal media.
- At DIV 10, replace the media with 1 ml of neuronal secretion media (see Recipes).
- Collect the media after overnight incubation; concentrate and analyze the media as in Procedure A.
- Lyse the cells directly in 500 µl of 2x Laemmli buffer.
- For representative examples of the results obtained using this protocol, please see Trajkovic et al., 2017.
- Coat each well of 6-well plates with 2 ml of poly-D-lysine (50 µg/ml in 0.05 M borate buffer [see Recipes]) overnight (RT). Wash each well with 2 ml autoclaved Milli Q water 1 x 5 min, 1 x 1 h, 1 x overnight, then 2 x 1 h on the next day (RT). Do not let the plates dry at any time. Fill each well with 1 ml serum media (see Recipes).
Data analysis
The intensity of bands was determined using ‘Analyze gels’ module of ImageJ software. The ratio between the extracellular and intracellular protein was determined. All experiments were repeated at least three times. Significance was determined using unpaired t-test.
Notes
Since proteins present in the cell culture media may be bona fide secreted proteins as well as proteins released due to spontaneous cell lysis and toxic effects of some reagents used to treat the cells, it is advisable to perform an LDH cytotoxicity assay on the media collected from control and treated cells. To that end use LDH Cytotoxicity Assay Kit according to manufacturer’s instruction. Only treatments that do not cause significant toxicity should be considered for further analysis.
Recipes
Note: All solutions should be stored at 4 °C.
- 0.05 M borate buffer
1.24 g boric acid
1.90 g borax
Dissolved in total 500 ml Milli Q H2O
Adjust pH to 8.5 using 1 N NaOH; filter sterilize - DMEM/10% HI FBS
Add 50 ml of heat-inactivated FBS to 450 ml DMEM - 0.3 M HEPES
7.15 g HEPES
~90 ml H2O
Adjust pH to 7.3 using 10 N and 1 N NaOH
Add Milli Q H2O to 100 ml final volume - HBSS
50 ml 10x HBSS
16.5 ml 0.3 M HEPES (see above)
5 ml 100 mM sodium pyruvate
3 g D-glucose (Sigma-Aldrich)
5 ml penicillin/streptomycin stock
Add Milli Q water to the final volume 500 ml; filter sterilize - Serum media
44 ml Neurobasal media
5 ml HI FBS
0.5 ml penicillin/streptomycin stock
0.5 ml glutamine stock - Full neuronal media
10 ml B27 supplement
5 ml penicillin/streptomycin stock
5 ml glutamine stock
Added to 480 ml Neurobasal medium - Neuronal secretion media
1 ml B27 supplement
5 ml penicillin/streptomycin stock
5 ml glutamine stock
Added to 489 ml Neurobasal medium
Acknowledgments
This work has been supported by R01NS080331 (D.K.). This protocol has been originally published in Journal of Neuroscience (Trajkovic et al., 2017). The authors declare no conflict of interest.
References
- Chevallet, M., Diemer, H., Van Dorssealer, A., Villiers, C. and Rabilloud, T. (2007). Toward a better analysis of secreted proteins: the example of the myeloid cells secretome. Proteomics 7(11): 1757-1770.
- Lee, M. C., Miller, E. A., Goldberg, J., Orci, L. and Schekman, R. (2004). Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20: 87-123.
- Momen-Heravi, F., Balaj, L., Alian, S., Mantel, P. Y., Halleck, A. E., Trachtenberg, A. J., Soria, C. E., Oquin, S., Bonebreak, C. M., Saracoglu, E., Skog, J. and Kuo, W. P. (2013). Current methods for the isolation of extracellular vesicles. Biol Chem 394(10): 1253-1262.
- Trajkovic, K., Jeong, H. and Krainc, D. (2017). Mutant huntingtin is secreted via a late endosomal/lysosomal unconventional secretory pathway. J Neurosci 37(37): 9000-9012.
- Zhang, M. and Schekman, R. (2013). Cell biology. Unconventional secretion, unconventional solutions. Science 340(6132): 559-561.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Trajkovic, K., Jeong, H. and Krainc, D. (2018). Mutant Huntingtin Secretion in Neuro2A Cells and Rat Primary Cortical Neurons. Bio-protocol 8(1): e2675. DOI: 10.21769/BioProtoc.2675.
- Trajkovic, K., Jeong, H. and Krainc, D. (2017). Mutant huntingtin is secreted via a late endosomal/lysosomal unconventional secretory pathway. J Neurosci 37(37): 9000-9012.
Category
Neuroscience > Nervous system disorders > Cellular mechanisms
Biochemistry > Protein > Immunodetection > Western blot
Cell Biology > Cell-based analysis > Protein secretion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link