- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Sensitive Estimation of Flavor Preferences in STFP Using Cumulative Time Profiles
Published: Vol 7, Iss 21, Nov 5, 2017 DOI: 10.21769/BioProtoc.2601 Views: 7077
Reviewed by: Soyun KimAlexandra GrosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2606 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2571 Views
Abstract
Social transmission of food preference (STFP) is observed among rodents between a demonstrator and a naïve hungry observer. During social interaction, hungry observer receives information about safety of the food consumed by the demonstrator. This task has been implemented to develop a single trial non-aversive learning task in order to test hippocampus dependent non-spatial memory in rodents. In this protocol, we describe some novel modifications to the conventional STFP protocol and analysis for more sensitive estimation of change in preferences. Using this method, preference trends can be observed for weeks after training, allowing one to probe the role of systems consolidation (SC) in declarative memory that is relatively independent of spatial navigation.
Keywords: Remote memoryBackground
Bennet G Galef Jr. developed STFP based behavior paradigm with rats during 1970’s in order to test memory mechanisms and since then, it has been implemented in various studies with both rats and mice (Galef Jr, 1977; Clark et al., 2002; Wrenn et al., 2003; Ross and Eichenbaum 2006; Smith et al., 2007; Choleris et al., 2011; Lesburguères et al., 2011; Clark, 2012). In case of rodents, the basic premise of this paradigm comes from their natural feeding behavior. Demonstrator mice [DemoMice] find the food that is safe for consumption through trial and error, and soon after consumption, the information for palatable food is shared with other observer mice [ObMice] through social interaction. Such interactions happen at a location situated away from the feeding site where ObMice learn about the safety of consumed food, or more specifically consumed flavor, when it is detected along with certain breath components of the DemoMice (Galef et al., 1988; Choleris et al., 2009). After establishing such flavor-safety association in STFP paradigm, ObMice have been observed to preferably consume the demonstrated flavor (demoFlavor), when given a choice between a novel and a familiar flavor.
Traditionally, the experimental design would involve arriving at the flavor pairs where both flavors are equally palatable during a consumption session with close to 50% preference for each flavor (Galef and Whiskin, 1998). Since the relative palatability of flavors is determined with different set of animals, one does not get any direct information reflecting innate preferences (IP) of experimental animals. After determining relative palatability of the flavor pair, one of these flavors is demonstrated through social interaction and increase in its preference beyond the 50% level is estimated after STFP. However, such a design does not consider the fact that even though average preference of a group of mice could be 50%, there could be individuals with varying native preference and this in turn could bias the interpretations of the result. Our novel design measures the STFP mediated change in preference while considering the native preference of individual animals.
Further, conventional preference estimation involves comparing the weight of food containers recorded before and after the sessions to calculate ‘weight of consumed food’ (WC.F). During a typical STFP testing session with mice, each individual consumes ~1 g and spills the food weighing up to ~5 g from containers weighing close to 100 g (typical weight of the container had to be ~100 g to prevent toppling). Correcting for errors associated with spillage limits the accuracy of WC.F. Consequently, it makes it difficult to detect minor changes in the strength and nature of the flavor-safety associations. Alternatively, we propose and utilize the ‘number of food consumption episodes’ obtained through video analysis as a measure of performance. Using this method, we establish an STFP procedure that is easy to implement and more sensitive. Inherently, such analyses are easy to use and less error prone as compared to weight measure. They utilize more data points and hence they convey more information in comparison to single point measurements such as total weight of consumed food or total time spent during consumption. Since mice consume small amounts over extended time windows, food intake data cannot be used directly for observing variations in rates of food consumption.
Materials and Reagents
- Identical plastic containers (Figures 1A and 1B): Dimensions 6.5 x 4.5 cm, assembled weight ~100 g (Julia Pet Jar 130 ml) (Princeware, catalog number: 9462 )
- Bedding material made from non-uniform corn cobb granules of 3-4 mm average diameter (Spar Cobb, Sagar Industries, Bangalore, India)
- Food vessel: A lid for smaller plastic container was used as food vessel (Figure 1) (Julia Pet Jar 60 ml) (Princeware, catalog number: 9461 )
Note: It is filled with ground flavored food and placed above the bedding material inside the plastic container. The lid of plastic container is closed such that the centers for food vessel and hole in the lid are aligned vertically. - C57/B6 mice (males, 8-12 weeks of age) housed in pairs after weaning until the beginning of habituation sessions. In order to clearly identify observer mice from demonstrators during interaction sessions in our experiments, demonstrator mice are marked with one hole in each ear pinna under general anesthesia after one week of weaning. Animals are separated for individual housing just before first habituation session.12-16 mice per group provide acceptable power during statistical analyses. The mice are reared in 12 h light/dark cycle and all the experiments are conducted during the light-on phase (06:30 AM-18:30 PM). All the procedures involving animals were performed with approval from institute animal ethics committee, IISc Bangalore
Note: One important consideration is co-housing of observer mouse with demonstrator mouse after weaning. In our method, we do not use a wire mesh/screen during social interaction to separate ObMice and demoMice, in order for the mice interaction to resemble their natural setting. To avoid excessive fighting during social interaction, we randomly select two just-weaned mice and co-house them in the same cage for one month. At the age of ~2 months, these mice are separated to be housed individually for further steps of the protocol and one of them is assigned to become a demonstrator for its cage-mate. Social interaction between two familiar male mice may also help in making it more effective for STFP in comparison to interaction with a stranger male. So, it is crucial to collect a large number of mice in comparable age group at the same time. Either observer or demonstrator mouse can be marked by making a hole in their ear pinna in order to identify them correctly after social interaction session. - Mouse food pellets (Nutrilab Rodent Feed, Provimi)
- Powdered condiments as flavoring agents (Cocoa, Cinnamon, Thyme, Basil; SNAPIN herbs and spices, Lotus household product, India)
Note: Source of all the condiments must be consistent from the beginning to the end of the experiment. - 70% ethanol to clean all the components of food apparatus
Note: Ethanol cleaning is carried out a day before the experiment session. All clean components are dried in warm air flow overnight to remove any odor trace from ethanol. - Sodium hypochlorite solution (4% NaOCl solution) (Fisher Scientific, catalog number: SS290-1 )
Note: It is further diluted to make cleaning solution (see Recipes) for removing prevailing odor from the components of food apparatus and animal cages. After each experiment session, all the components of food apparatus are submerged in a cleaning solution for 15-20 min followed by thorough rinsing in tap water. Cleaned components are then air dried and stored in hygienic conditions. - X% ‘Condiment’ flavored food (see Recipes)
- Cleaning solution (see Recipes)
Equipment
- Plier for making metal trays
- Paint for metal trays: white paint if black coat mice are used and vice versa
- Transparent Perspex sheets with holes drilled along short central axis (Figures 1E)
- Aluminum/Tin metal sheet (sized to fit in the test cage): 1-2 mm thickness for making spill-proof trays to hold food containers. The vertical walls of these trays are 2 cm high. Length and breadth of these trays can be adjusted for achieving best fit within the test cage. For our setup, the dimensions were 16 x 13 cm
- Weighing balance: with sensitivity up to 10 mg
- Electronic grinder (Morphy Richards, model: Icon Essentials ) for making powdered food
- Full HD webcam (Logitech, model: C920 ) for multiplexed video monitoring
- Polarizer filter (RG610, RG series color glass filters, Optica, Optics India; it is optional) to avoid reflections from transparent Perspex sheets covering the test-cages
- Webcam mounting assembly: We used a long wooden stick (300 x 10 x 5 cm3) with a hole at the center along its length and an M6 screw to fix the webcam above test-cage assembly
- Individually ventilated cages (IVCs)/polycarbonate cages for individual housing of mice (IVC; 36 long x 14 wide x 12.5 cm high) (Citizen Industries, catalog number: 11 )
- Portable electronic drill (Robert Bosch, model: Bosch GSB 10 RE Professional ) with drill bits to make holes of 1 cm diameter in the lids of food container
Software
- Free, open source media player such as VLC. Any software for position tracking may also be implemented for estimating time spent near food containers
- Origin software
- Microsoft Excel
- ImageJ plugins (such as Analyze particle)
Procedure
- Preliminary requirements
- Design of feeding apparatus (Figures 1A and 1B): This includes a cylindrical food container with a hole in its lid, a small food vessel and an aluminum tray for collecting spilled food.
- Identical plastic containers must be used. Lids should be sufficiently flat for mouse to sit on top and consume food.
- The lid of each plastic container is drilled at the center to make a hole of 1 cm diameter. Holes must be of the same diameter in all the containers.
- Bottom container is filled with bedding material leaving ~1 cm at the top.
- A small cylindrical food vessel of ~1 cm height is filled with powdered food and kept inside the bottom container.
- The lid is tightened on top of the food vessel aligning their centers vertically. Food should be accessible through the hole in the lid.
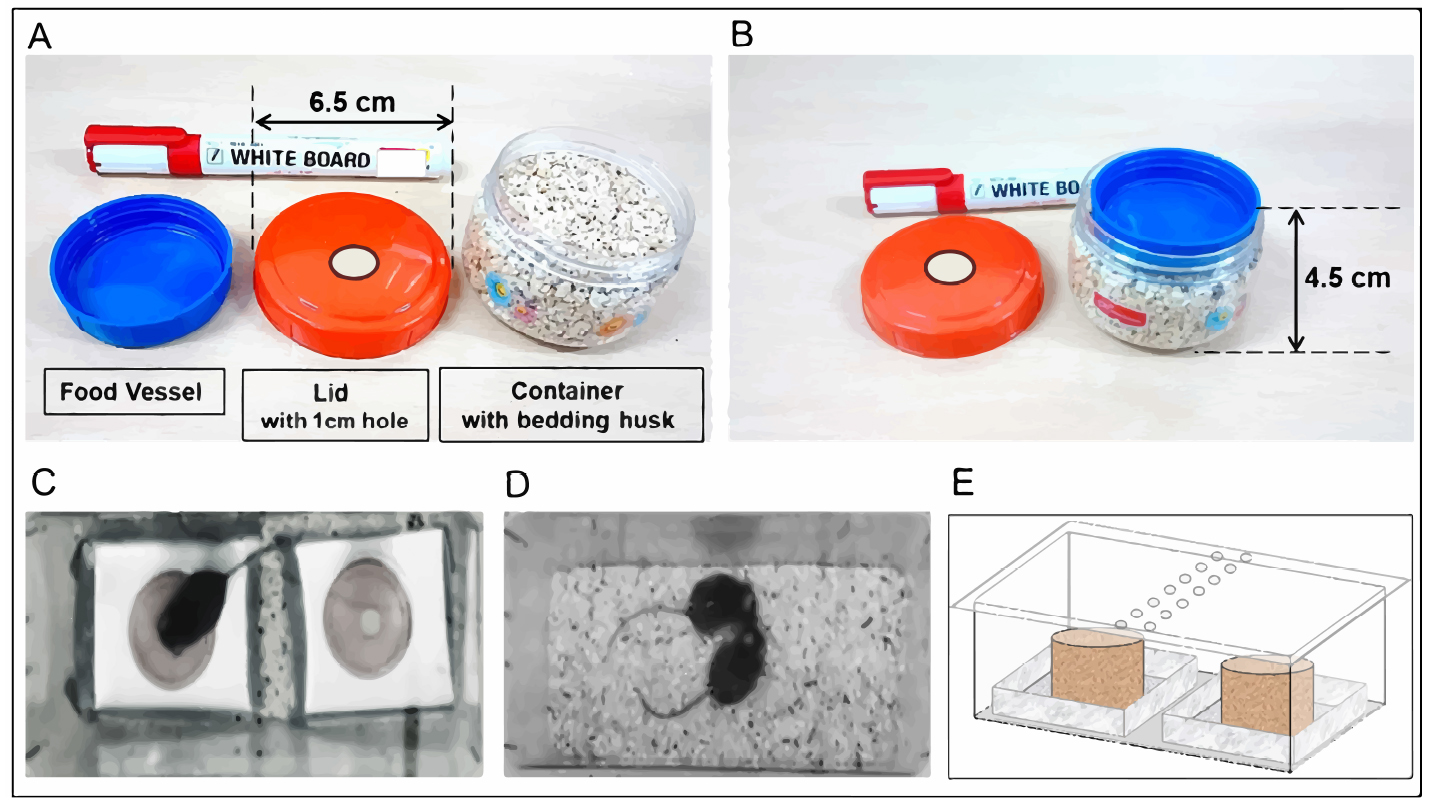
Figure 1. Design of food apparatus (A, B) used during food consumption session (C); During food consumption and social interaction session (D), the test cages were covered with a transparent Perspex sheet (E). All observer mice are provided with powdered food in two identical sets of food apparatuses during preference tests. A. Components of food container include a food vessel filled with powdered food (powdered food not shown) that rests within the bottom container. The lid is modified by drilling a hole of 1 cm diameter at its center to provide access to food. B. Bottom container is filled with bedding material leaving just enough space to place the food vessel on top. C. Mice consuming food from one of the food containers during a preference test. Both food containers are identical in every aspect of their design. If spilled food gets mixed with bedding material in the cage, it is extremely hard to account for during estimation of net consumption. To minimize such unaccountable spill during consumption, each feeding apparatus includes a custom-built aluminum tray to hold the food containers. The food containers are softly glued to the center of the metal tray with a small piece of double-sided adhesive tape. D. Social interaction between observer and demonstrator mice. Demonstrator mice are identified by a hole in their ear pinna. E. Side view of a test cage covered with a transparent Perspex sheet to avoid mice from escaping while video monitoring. Two rows of holes meant for air circulation are visible close to the center of transparent Perspex sheet covering the cage. - A small piece of double-sided adhesive tape (1 x 1 cm) is attached to the center of spill collection tray. After weighing the food containers, they are fixed to the spill collection tray. Spill collection tray was made by bending thin metal sheet. Its dimensions can be decided according to the test cage.
Note: Size and position of feeding hole on all the lids need to be same, in order to make sure that accessibility to food is uniform for all mice. For black coat mice, all the spill collection trays and lids for food containers can be painted white to provide the best contrast for video analyses. - Containers are temporarily labeled at the bottom with their flavor and their weights are noted with respect to their position in each test cage.
- Identical plastic containers must be used. Lids should be sufficiently flat for mouse to sit on top and consume food.
- Cage assembly and video recording set up
- Test cages with bedding material are arranged in a matrix on the floor/low table in the experiment room. Depending on the number of mice, one could use 3 x 3, 3 x 4 or 4 x 4 matrix to arrange test cages.
- It is important to place visual block between all the transparent/translucent cages in order to avoid visual distractions across mice. We placed cardboard sheets (15 cm high, 3 mm thick, length as per the cage arrangement) to maintain complete visual block between test cages during the experiment.
- Test cages are covered with transparent Perspex sheets (Figure 1E) for multiplexed video recording of all the cages in top-view (Figures 1C and 1D).
- Each Perspex sheet has small holes (3-5 mm diameter) drilled along the short central midline for air circulation (Figure 1E).
- A webcam is mounted at ~4 ft height using a customized stand such that each test cage is covering an equivalent area in the field of view and all cages are in focus.
Note: Any reflection from transparent Perspex cover on test-cages can be avoided by using well-directed lighting and/or a polariser.
- Test cages with bedding material are arranged in a matrix on the floor/low table in the experiment room. Depending on the number of mice, one could use 3 x 3, 3 x 4 or 4 x 4 matrix to arrange test cages.
- Design of feeding apparatus (Figures 1A and 1B): This includes a cylindrical food container with a hole in its lid, a small food vessel and an aluminum tray for collecting spilled food.
- Steps for the procedure
Our STFP behavior protocol has been explained in ten steps including 1) Weaning, 2) Random paired co-housing of two mice in single cage, 3) Handling, 4) Segregation of paired mice as observer and demonstrator, 5) Habituation (5-7 days), 6) Pre-STFP preference test (1 day) for Identification of less preferred flavor as to-be-demonstrated flavor, 7) Feeding respective demonstrator mice on to-be-demonstrated flavor, 8) Social interaction (1 day), 9) Post-STFP preference tests (1 day each) at recent time-points, 10) Post-STFP preference tests (1 day each) at remote time-points (Figure 2). Pre-STFP preference, Social interaction and Retrieval tests were all performed in the same room but on different days. All animals were housed in individually ventilated cages to prevent the unwanted transfer of olfactory, auditory or visual cues between demonstrator and observer mice across sessions. If individually ventilated cages are not available, holding and testing rooms must be separate. Clean-air circulator and exhaust outlets were kept open continuously to prevent odor retention of previous sessions. Clean-air inlet was turned off during the experimental sessions for one hour.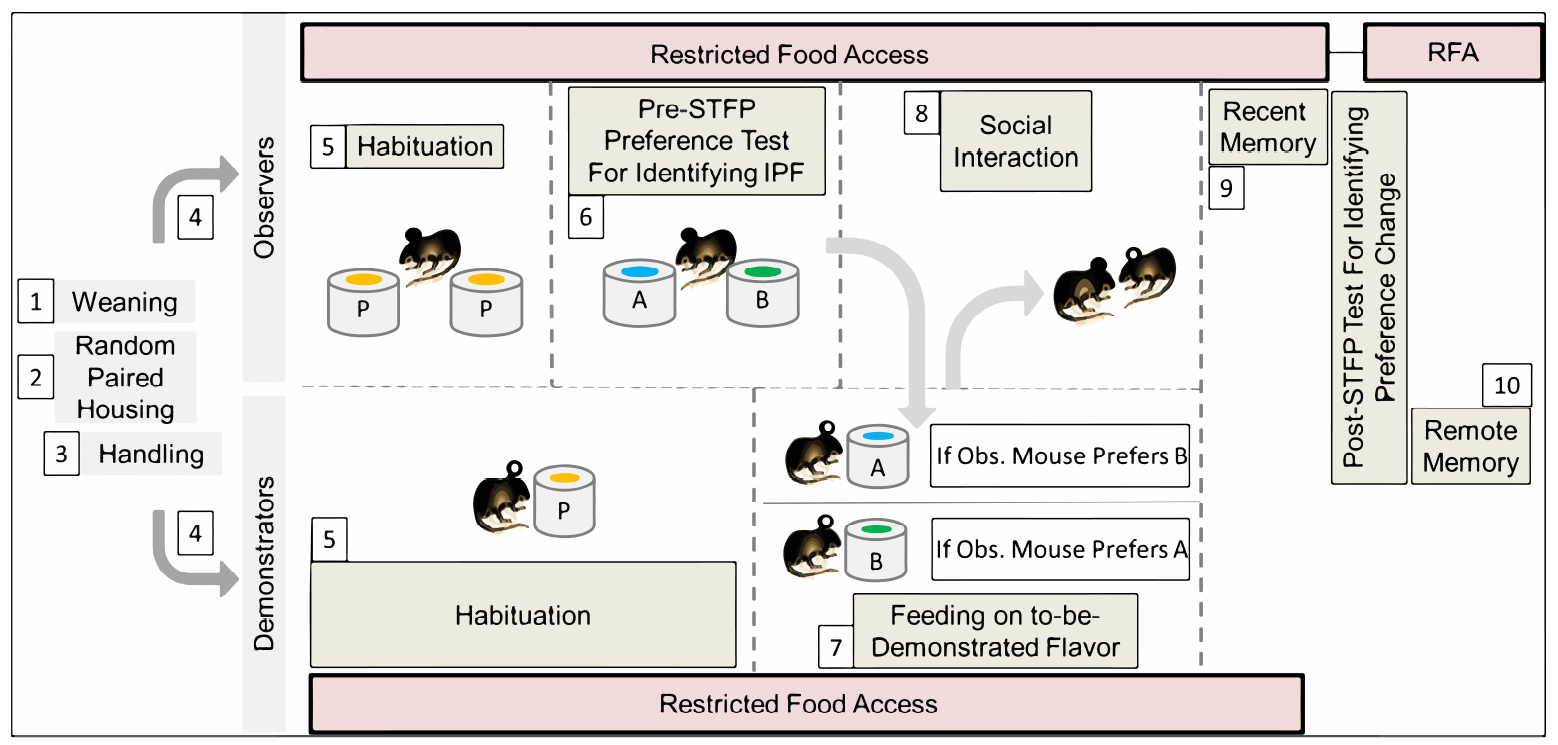
Figure 2. Modified protocol for STFP with mice. Each step is numbered and explained in detail in this procedure section. P–plain powdered food (without added flavor), A, B–flavors used during the experiment, IPF–innately preferred flavor, RFA–restricted food access (mice were provided with 1 g food pellet daily in addition to the consumption during habituation/testing sessions).- Weaning
3-4 weeks old male mice are separated from the mother based on their size and health. - Randomly paired housing
In our protocol, paired housing begins with weaning. Randomly paired two animals are co-housed in a cage just after weaning and kept together until the beginning of habituation sessions. Habituation sessions begin after a minimum of four weeks from weaning. A week after weaning, one of the co-housed animals is ear marked to be the demonstrator. - Handling
Co-housed animals are handled for about a week before separation. Proper handling helps in reducing anxiety in both animals and the experimenter during behavior. Handling protocols followed in different labs are mostly similar with few modifications depending on the cohort. We are listing down handling steps which we followed while considering the anxiety levels of both the novice animals and the experimenter.
Note: Each animal is handled for ~10 min daily for 3-5 days in the room where the experiment will be conducted.- Day 1
Gently lift the animal while holding the tail closer to the body. Rest the body on other hand for 20-30 sec while both the hands are within the home cage. Release back in the cage gently. Repeat 3-5 times at an interval of ~30 sec depending on anxiety of the animal.
Note: Animals within a cage can be handled with same pairs of gloves as they are familiar with each other’s odor. It is important to change gloves while handling animals from a new cage. Gloves should be changed across cages even if there is no/minimal excretion from previous cage animals. - Day 2
Repeat day 1 procedure while resting the animal for 60-90 sec on palm. Always change gloves for seemingly anxious animals even if they are cage mates of relatively non-anxious animals. - Day 3
Repeat day 2 procedure while allowing the animal to walk around for ~2-3 min while positioning the palms just above the cage. Try to make sure that animal lands in cage if it jumps. Follow same glove change rule as of day 1 and day 2.
Note: In case animal jumps and lands out of the cage (on floor/table), gently lift the animals, keep them back in cage and resume handing only the next day for such animals. Prior arrangements should be made to make all the hiding corners inaccessible for the loose mice. - Day 4/5
Repeat the steps followed on day 3. If animals seem anxious or ‘jumpy’, repeat over following days. Usually, most of the animals get rid of their anxiety within 4-5 days of handling.
- Day 1
- Separating Co-housed animals: Co-housed animals are separated and housed individually in different cages just before the beginning of habituation sessions. The same couple needs to be used during all the interaction sessions to avoid fighting and stress. All the individually ventilated cages for holding single animals are kept in the same room.
- Habituation
Habituation allows animals to get adjusted with two main aspects of the paradigm: daily food deprivation of ~15 h and consuming powdered food pellets by climbing on custom-made food apparatus.
Food Deprivation: Food deprivation involves limiting access to food by providing animals with a 1 g solid food pellet daily, in addition to the one-hour food consumption session every day. Food deprivation begins by leaving animals with just a 1 g solid food pellet 24 h before commencing the first habituation session for one-hour of food consumption. Food deprivation schedule is continued from the beginning of habituation sessions until the post-STFP preference test conducted after 24 h. For 17- and 41-day remote preference tests, ~15 h deprivation is started 3 days in advance before the testing day. Water is provided ad libitum, except for one hour of experimentation/habituation session.- Preparation, distribution and weighing of food: Regular food pellets are ground before each session. Food was prepared in a ventilation hood with minimum air flow settings.
- Each food vessel is filled with powdered pellets and enclosed in the food container.
- Weight of each container is carefully noted down in accordance with its relative position with respect to the test room as frame of reference.
- Each food container is kept in a light metal-tray custom built for collecting any spilled food.
Note: Weights of food containers are noted before and after the session. Spilled food is weighed indirectly as explained in the ‘weighing procedure’ below. - Demonstrator mice are given feeding-apparatus within their home cage and allowed to consume food for one hour.
- Observer mice are first released in the test cage at their respective position. Then two sets of feeding-apparatus are introduced one after another. Some important considerations in order to avoid biased food-distribution are listed in the note below.
Note: During habituation, each feeding-apparatus is identical in every aspect including flavor of contained food. So, only variable remains the order of delivery. We change the order of delivery across cages within the same session as well as across sessions within the same cage. For example, on day 1 of habituation, if cage 1 first receives food at position A and then at position B, we ensure that on day 2, position B is given food before position A. Also, within the same session, we ensure the order apparatus placement is alternated across cages i.e., if cage 1 receives food first at position A and then at position B, we ensure that cage 2 receives food first at position B and then at position A. This pseudo-randomization doesn’t allow mice to prefer any peculiar end within the test cage based on ‘first come, first eat basis’ and makes it easier for them to learn to eat equally from both containers. - Weighing Procedure: Weights of food containers are noted before and after the session. Spilled food is weighed indirectly. Any cage litter is removed before weighing the tray. The tray containing spilled food is weighed after the session and its weight is noted. Then the tray is wiped thoroughly to remove any sticking powdered food and weighed again. The difference in these two weights of the tray is noted as ‘spilled food’ and subtracted from the difference of ‘before and after’ weights of the food container to obtain ‘net consumption’. Preference for the flavor A is calculated as the ratio of net consumption from container A vs. total food consumed from both the cups during one-hour session.
- Each food vessel is filled with powdered pellets and enclosed in the food container.
- Day 1
Demonstrator mice: They are provided powdered food in a container in their home cage for 1 h. DemoMice are given food one hour before ObMice. Water is removed during food consumption.
Observer mice:- ObMice are individually released in their respective test cage containing bedding material for 1-h long consumption session.
- Trays containing food container are then placed in each cage while following aforementioned pseudo-randomization process.
- At the end of the session, all mice are removed from test cages without disturbing the feeding apparatus and kept back in their home cages.
- All mice are given a solid food pellet weighing ~1 g after the session. This results in daily food deprivation of ~15 h.
- Feeding apparatuses are individually removed from each test cage, carefully weighed in aforementioned manner to calculate net consumed food and dismantled for a thorough cleaning that involves cleaning with odorless detergent/bleach, washing with warm water and drying in warm air flow.
- ObMice are individually released in their respective test cage containing bedding material for 1-h long consumption session.
- Days 2-7
Same timing is maintained for feeding both DemoMice and ObMice. It is repeated until observer mice begin to consume equal amount of food from both food containers. Position of containers within a test cage is reversed across sessions over multiple days.
- Preparation, distribution and weighing of food: Regular food pellets are ground before each session. Food was prepared in a ventilation hood with minimum air flow settings.
- Pre-STFP preference test
- Preparation, distribution and weighing of Food:
- Just before commencing the test session, powdered food pellets are mixed in a required ratio (% w/w) with the commercially acquired flavors. See ‘Recipes’.
- During preference tests, freshly prepared food of one flavor is filled in the food vessel which is then kept inside the container. Containers with the same flavor are assembled first.
- The place is thoroughly wiped to remove any traces of previous flavor and remaining vessels are filled with the powdered food containing other flavor and remaining containers are assembled.
- Steps ‘i to v’ as for observer habituation are followed further.
- Weighing is conducted in aforementioned manner.
- Just before commencing the test session, powdered food pellets are mixed in a required ratio (% w/w) with the commercially acquired flavors. See ‘Recipes’.
- ObMice are allowed to consume flavored food from two containers in the test cage kept at their respective positions in the assembly. Complete session is video recorded in addition to the weight measurements.
- Based on the weight estimation, each observer mouse is identified as preferring flavor A or flavor B. Choice of flavor at this point most likely represents their innate preference for one of the two flavors. It is noted that during social interaction after 24 h of pre-STFP test, each mouse preferring flavor A during pre-STFP test will be demonstrated with flavor B and vice versa. Since preference for either flavor ranges between 0-1, ObMice having more than 0.5 preference for flavor A will be demonstrated with B and vice versa.
- There might be some ObMice which consume equal amounts of both the flavors during pre-STFP test. Half of these mice are allotted to flavor A preferring group and the other half to flavor B preferring group.
Note: In our experiments, steps 7 (DemoMice feeding) and 8 (social interaction) were most often carried out after 24 h of step 6 (Pre-STFP test). We also tested a scenario when steps 7 and 8 were conducted five days after step 6. Animals need to be given 2 g food pellet daily during the intervening days for maintaining a healthy weight.
- Preparation, distribution and weighing of Food:
- Demonstrator Feeding: Respective demonstrator mouse from the co-housed pair is fed for 1 h on the flavor which is preferred less during pre-STFP test by the corresponding observer. This feeding takes place in the individually ventilated home cages of demonstrator mice.
- Social interaction for demonstration of flavor (STFP)
- At the end of feeding session, demonstrator mice are released in the test cage of corresponding, pre-assigned observer mice for one hour long social interaction.
- All mice are kept back in their respective home cages at the end of interaction session.
- Interaction session is also video monitored.
- At the end of feeding session, demonstrator mice are released in the test cage of corresponding, pre-assigned observer mice for one hour long social interaction.
- Recent memory test
In order to test the change in preference after social interaction, 24 h after social interaction observer mice are provided both the flavors in two cups in their respective test cages (see Video 1). Post-STFP tests follow the same steps as required for pre-STFP preference test. - Remote memory test
Remote memory for flavor retention can be carried out after 2-4 weeks depending on the requirement.Video 1. Food consumption in a typical test-session conducted after 24 h STFP
- Weaning
Data analysis
- Preference estimation using weight measurement
- In order to quantitatively express the demoFlavor preference w.r.t either weight or time as a measure, we define mean preference as follows:
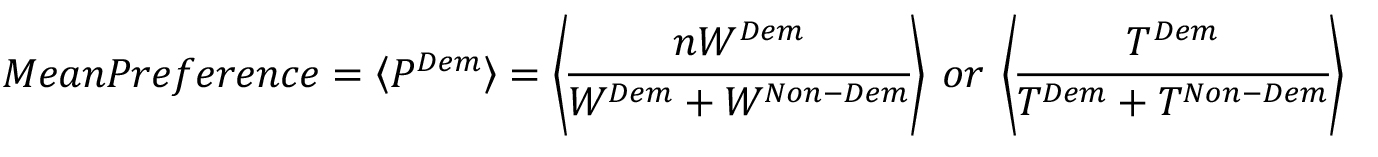
where, WDem and WNon-Dem are Consumed weight of demoFlavor and non-demoFlavor, respectively; TDem and TNon-Dem are Time spent near demoFlavor and non-demoFlavor container, respectively.
- In order to quantitatively express the demoFlavor preference w.r.t either weight or time as a measure, we define mean preference as follows:
- Preference estimation using time spent near food cup
- Video recording
All the food consumption sessions were video recorded at 30 frames per second. These videos are then manually scored to record the position of each mouse at an interval of every 10th sec (300th frame). Steps for manual scoring are listed below. - Manual scoring
- A session starts after both the food containers are placed in the test cage with mouse. Each video is continuously observed with intermittent pause after every ten sec.
- As mentioned before, the videos are recorded at 30 fps, all cages are continuously observed and mice positions are noted after every 10 sec i.e., 300 frames. This results in each 1 h long video of 3,600 sec to be scored to give 360 data points for each mouse. Scorer should be blind to the information about flavor in food cups while scoring.
- Each cage (C1, C2 etc.) is assigned 2 columns in the manual scoring sheet (Figure 3A, see the sample excel sheet here) for recording position of mice while scoring.
- One of the ways to keep track of manual scoring could be as follows: If the animal is in left half of the cage, then ‘a’ is diagonally crossed. If the animal is in right half of the cage, then ‘b’ is crossed. If animal is in center of the cage ‘©’ is written between a and b. To avoid marking mistakes, a and b for consecutive cages are printed in different fonts (Figure 3A). ‘a, b, and ©’ can be replaced with symbols of choice.
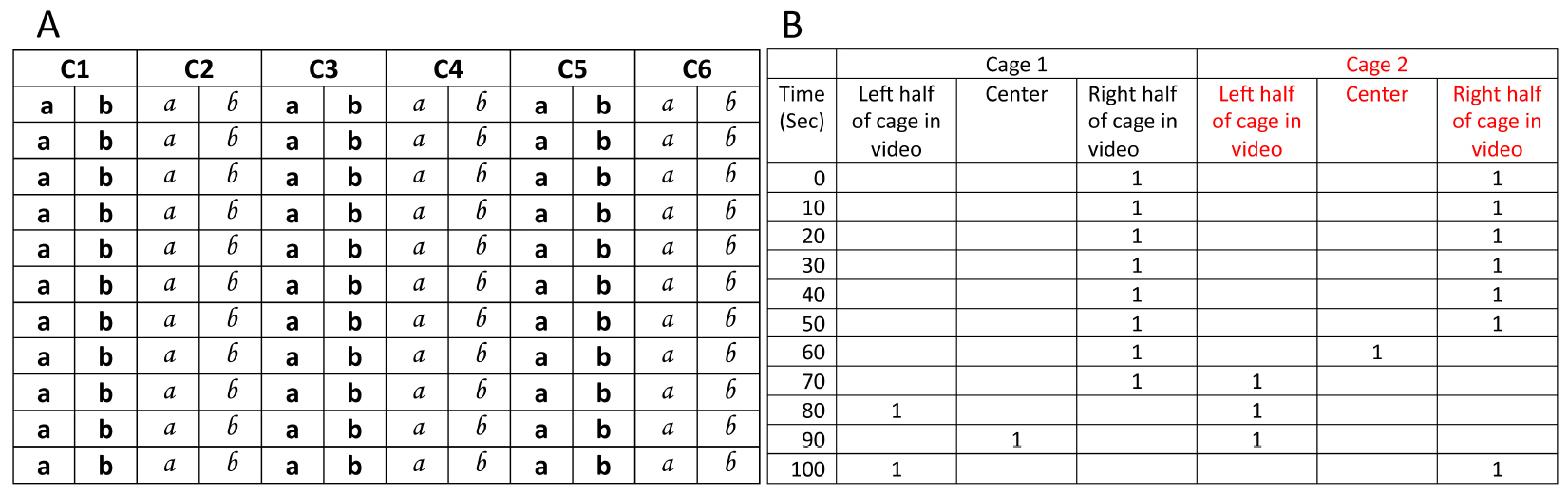
Figure 3. Portions from example sheet for manual scoring (left) and sample excel sheet for processing manual scores (right). A. C1 refers to cage 1; while manual scoring, video is paused after every 10 sec and position of mouse in cage is recorded (see step ‘d’ in manual scoring above). B. After manual scoring is complete, positions of mice are recorded in analysis sheet. For first 10 sec window (first row marked with time ‘0’), if mouse is found to be in right half of the cage, crossed ‘b’ is recorded by entering ‘1’ in the column titled ‘Right half of cage in video’ and so on. After translating all the data to analysis excel sheet, a macro is run for identifying eating episodes. Cumulative eating episodes for subsequent time windows are then plotted to generate cumulative time profiles.
- A session starts after both the food containers are placed in the test cage with mouse. Each video is continuously observed with intermittent pause after every ten sec.
- Automated scoring
We are in the process of developing a fully automated method to estimate individual flavor preferences using ImageJ plugins (such as Analyze particle) to identify the size of the mice. We have generated heat maps of mice to represent their preferences for each location. We are in process of incorporating the algorithm for identifying eating episodes based on heat maps. We will further extend it to generate live cumulative time profiles as the session progresses.
- Video recording
- Processing raw data from manual scoring
- All mice positions from the scoring sheet are entered in the Excel data sheet for further analyses (Figure 3B). For each cage, excel sheet contains 3 columns representing left, center and right region of the cage and, 360 rows of cells each representing 10 sec of the video. After manual scoring is complete, positions of mice are recorded in analysis sheet.
Example: For first 10 sec window (first row marked with time ‘0’), if mouse is found to be in right half of the cage, crossed ‘b’ is recorded by entering ‘1’ in the column titled ‘Right half of cage in video’ while other two neighboring cells are left empty. When mouse position cannot be assigned to either left or right half of the cage, 1 is entered in the cell corresponding to the column marked as ‘Centre’. - Total time in a cage region is 10 times the sum of all ‘1’ in a column (since each ‘1’ represents 10 sec). In our videos, we observed that mouse does not always consume food when they visit a region for short durations. We found that residence time was highly correlated to food consumption if we defined an eating episode to be of 30 sec or longer duration. Simply put, based on the correlation in addition to our observation during video monitoring, we can safely assume that mice were consuming food whenever they spent more than 30 sec at a location. Such durations which are either equal to or longer than 30 sec are now referred to as ‘eating episodes’. So, in order to calculate total consumption time spent near a food cup, using a visual basic macro we counted only those data points which occur as a consecutive bunch of three or more ‘1’ at the same location. In other words, for calculating total time at a location, only those visits are considered to be ‘eating episode’ when a mouse stays at a location continuously for 30 sec (900 frames) or longer. Instances when mouse position is marked to be in the center of the cage are not accounted for calculating preferences.
- After translating all the data to analysis excel sheet, a visual basic macro is run for identifying eating episodes. Cumulative eating episodes for subsequent time windows are then plotted to generate cumulative time profiles. Macro identifies and assorts information about time spent near a food container into ‘eating episodes’ (This macro is just an example for selecting 60 sec long episodes; it needs to be modified according to the duration of eating episodes, total duration of consumption session and number of cages):
Sub DataAnalysis ()
Dim row As Long
Dim column As Long
Dim sum As Integer
Dim newcol As Long
newcol = # (enter column # where you need data after running macro)
For column = # to # (enter column numbers where raw data is entered)
For row = # to # (enter row numbers where raw data has been entered)
sum = WorksheetFunction.sum(Sheets(‘Sheet1’).Range(Cells(row, column), Cells(row + 5, column)))
If sum = 6 then
Cells (row, newcol).Value = Cells (row, column).Value
Cells (row + 1, newcol).Value = Cells (row + 1, column).Value
Cells (row + 2, newcol).Value = Cells (row + 2, column).Value
Cells (row + 3, newcol).Value = Cells (row + 3, column).Value
Cells (row + 4, newcol).Value = Cells (row + 4, column).Value
Cells (row + 5, newcol).Value = Cells (row + 5, column).Value
Cells (row + 6, newcol).Value = Cells (row + 6, column).Value
End If
Next row
newcol = newcol + 1
Next column
End Sub - Time windows
We then compare the cumulative consumption of mice within eleven time-windows encompassing the one-hour consumption session. First window is 10 min long while subsequent ten windows are 5 min long each.
Note: After consumption session begins for first cage, it takes ~5 min to arrange things for food consumption in the last cage of the group leading to an additional 5 min delay in start of the session for last mouse. In addition, after initial switching between food cups, it takes a few minutes for mice to begin continuous consumption. Due to such delays, the first consumption window is 10 min long to obtain average food consumed and remaining 50 min of the session is divided into 10 windows of 5 min each. Average cumulative time spent near a food cup is used to generate cumulative time profiles (CTPs) for both demonstrated and non-demonstrated flavor.
- All mice positions from the scoring sheet are entered in the Excel data sheet for further analyses (Figure 3B). For each cage, excel sheet contains 3 columns representing left, center and right region of the cage and, 360 rows of cells each representing 10 sec of the video. After manual scoring is complete, positions of mice are recorded in analysis sheet.
- Cumulative time profiles (CTP) and Steps to generate CTPs from eating episodes
Using pattern of consumption as a diagnostic for predicting retention:
In case of rodents, meal microstructure can be analyzed using the food intake data obtained as a function of time during food consumption (Fox and Byerly, 2004). The food intake rates in such studies have been related to physiological parameters through Weibull function as follows: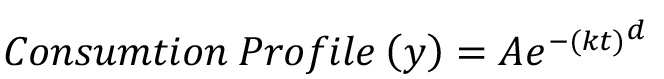
where, consumption profile (y) is obtained by measuring the amount of food consumed as a function of time. Such consumption profile has been found to depend on the three main parameters namely the initial intake rate (A), slope or rate of decline in intake rate (k) and deviation of the curve from the exponential (d) which represents the duration for which initial intake rates are maintained. One of the ways to interpret the Weibull fit parameters could be the following: parameter A may largely reflect how hungry the animal is at the beginning of the session, parameter k and d would mostly depend on innate preference for the flavor. These parameters may also have some contributions from the moisture content and stability of the food.
We extended this analysis for more effective monitoring of the average consumption profiles of animals during STFP paradigm. We plot cumulative eating episodes as a function of time to obtain the average consumption profile for group of mice. The Cumulative time profile (CTP) represents variation in the total number of eating episodes in successive 5 min-long time windows for an hour-long consumption session (details are explained in ‘Data analysis’ section). Therefore, the corresponding function to fit the CTPs is the integral of Weibull function i.e., Weibull cumulative distribution function (CDF) given by the following integral:
Parameters and their meaning: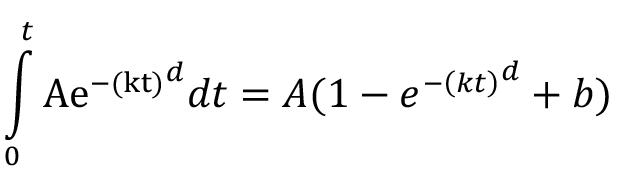
A–amplitude parameter representing cumulative consumption,
b–offset parameter representing the starting preference at the beginning of the session,
d–deviation parameter representing the deviation of fit from exponential,
k–slope parameter representing the decline in rate of consumption.
In a similar aforementioned manner, the WeibullCDF parameters could be interpreted as follows: parameter A may largely reflect animals’ hunger throughout the session, parameter k and d would mostly reflect change in preference for the flavors as session progresses.
Steps:- Amount of time spent in either half of a cage is measured in terms of eating episodes for each mouse.
- Eating episodes for each time window are summated for calculating cumulative time spent in food consumption. For each time window, cumulative time near each food cup spent is averaged for all the mice.
- Average cumulative counts for each time window are plotted against time in order to get a cumulative time profile for average time spent near both the food cups (Figure 4).
- CTPs are fitted to integral of Weibull function which we refer to as Weibull cumulative distribution function (WeibullCDF). One-way ANOVA is then used to compare the CTPs across successive sessions. Details of complete statistical analysis and fitting procedure have been explained in a recent study (Singh et al., 2017).
- Steps for fitting CTPs to Weibull Function: Origin software was used for fitting the consumption pattern data using linear and non-linear functions. Linear fit is provided with the package. For non-linear fit using Weibull function, we added the code in the function builder provided within origin package as follows:
Analysis → Fitting → Non-linear fit → Open Dialog → Create new fitting function → Select Category as ‘Growth/Sigmoidal’ → Name as ‘Weibull CDF’ (i.e., Weibull Cumulative Distribution Function) → Function Model set as ‘Explicit’ → Function type set as ‘Expression’ → Independent variable set as ‘x’ → Dependent variable set as ‘y’ → Parameters set as ‘A, b, d, k’ → Function body written as ‘A·(1 - exp(-(k·x)d)) + b’.
Note: While fitting CTPs to Weibull cumulative distribution function (WeibullCDF), parameter initialization values required for four parameters are as follows: A = 1,000, b = 100, d = 1, k = 0.01.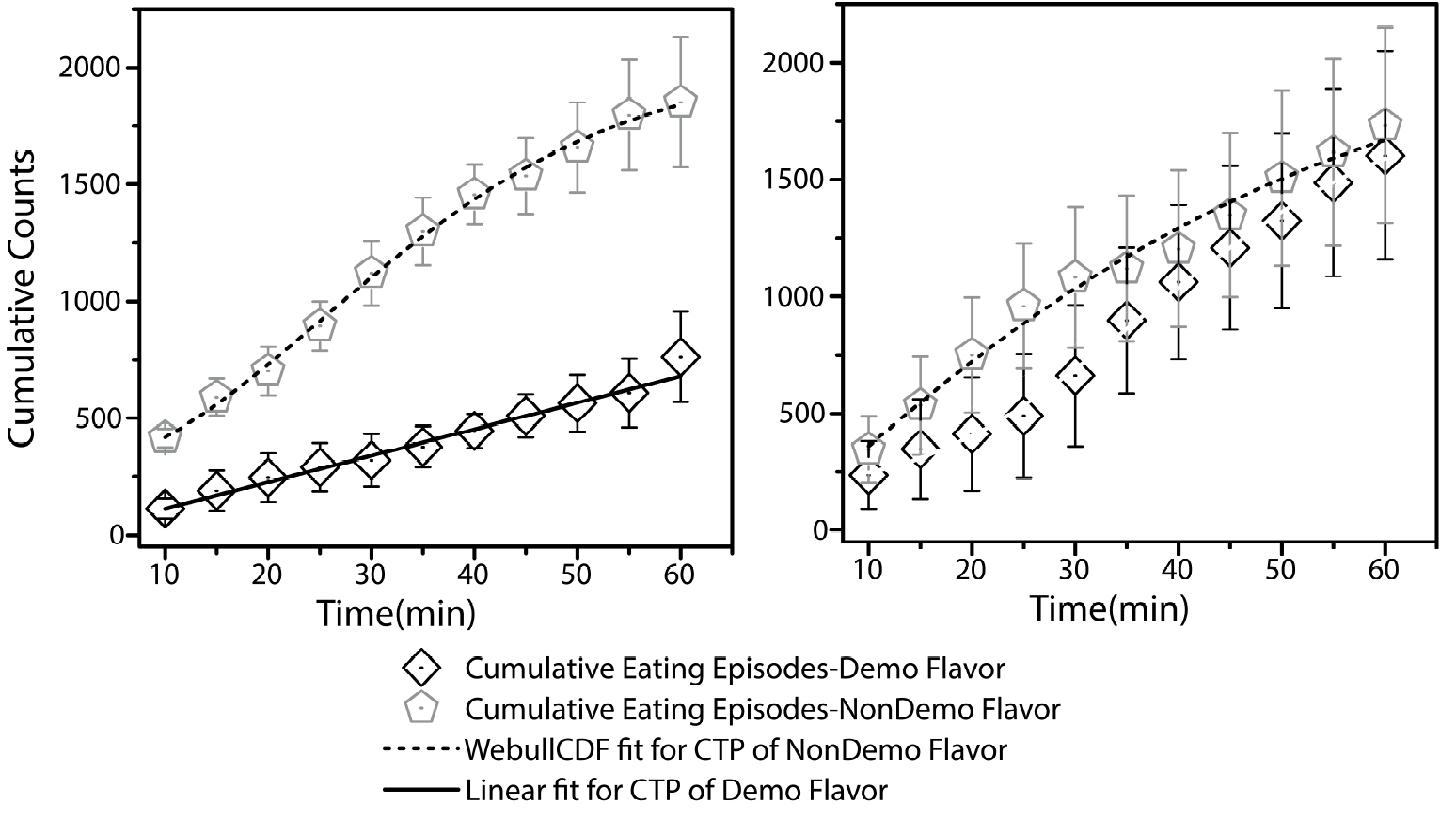
Figure 4. Cumulative time profiles (CTPs) representing consumption patterns for demoFlavor and non-demoFlavor for two preference tests: pre-STFP (left panel) and 24 h post-STFP (right panel). As indicated in the pre-STFP CTPs, flavor with lower preference (open diamonds) was selected for demonstration during social interaction. 24 h later, observer mice were demonstrated with less preferred flavor during social interaction with demonstrator mice that were fed on to-be-demonstrated flavor for one hour. Flavor with higher preference was not demonstrated. 24 h after STFP, CTP fits indicate the increase in preference for demoFlavor only. Both CTPs were fitted to Weibull cumulative distribution function (WeibullCDF). CTP fit for flavor B could also be approximated equally well by a linear fit. Each datapoint represents summation of eating episodes within respective time window. This data is part of the study that was published earlier (Singh et al., 2017). It is plotted here for representative purposes. Weight-based preference estimation data can also be accessed through the same study.
Note: At this point just after pre-STFP test in the actual experiment, only weight based consumption preferences are known whereas the time-based data is segregated into cumulative eating episodes after manual scoring of videos. Thus, less preferred flavor is identified as the flavor for which average weight of consumption is lower. In order to speed up the analyses, we are working on a heat map based method for cumulative eating episode based preference estimation.
- Amount of time spent in either half of a cage is measured in terms of eating episodes for each mouse.
- Simulation-based comparison of random vs. selective demonstration during STFP
Previous studies seldom considered the effect of innate preference during STFP behavior with rodents. In order to accommodate the contribution of animals’ innate preferences for evaluating the change in preference following demonstration, in our protocol we incorporated a pre-STFP test which is followed by ‘selective demonstration’ of flavors (Singh et al., 2017).
While conducting social demonstration after pre-STFP test, we had choice between two seemingly alternate ways of proceeding forward, i) selective demonstration: use the pre-STFP test data to group animals with similar preferences for demonstrating less preferred flavor or, ii) random demonstration: randomly distribute animals in two groups independent of pre-STFP test data for demonstrating any one of the two flavors. These two seemingly different approaches could result in introducing some artificial variations while estimating the change in preference across pre-STFP to post-STFP tests. To address this concern, we conceptualized virtual STFP where we simulated two preference tests and compared the effects of aforementioned flavor demonstration strategies on the change in preference. We found both demonstration approaches to be considerably similar based on the results of simulation and we proceeded with the first approach of selective demonstration.
For simulating STFP, we assume that preference estimated during each preference test has two major components, i) starting preference (SP) component for demoFlavor and, ii) random preference (RP) component for demoFlavor. Corresponding sets of SP and RP are assumed to have distributions with same mean (0.5) and standard deviation. Further, the contribution of both components is weighted with the parameter i, which represents the magnitude of contribution from natural bias or innate preference of animal for demoFlavor. Parameter ‘i’ ranges between 0 < i < 1.
The steps for simulations are as follows:- Generate first set of N random numbers with a mean of 0.5 and a fixed standard deviation (SD1). This set represents the distribution of ‘starting preference (SP)’ for N animals for demoFlavor. Each value represents SP for one virtual animal.
- Generate second set of N random numbers with a mean of 0.5 and fixed standard deviation as of first set (SD1). This set represents distribution of random preference component (RP1) of N animals for demoFlavor during virtual pre-STFP test.
- Generate third set of N random numbers with mean 0.5 and standard deviation SD1. This set represents distribution of random preference component (RP2) of N animals for demoFlavor during virtual post-STFP test.
- Evaluate the virtual pre-STFP preference (P1) by incorporating contributions from SP and RP1 weighted by the parameter ‘i’ using following formula:
P1 = i.SP + (1-i).RP1 - Evaluate the virtual post-STFP preference (P2) by incorporating contributions from SP and RP2 weighted by parameter ‘i’ using following formula:
P2 = i.SP + (1-i).RP2
Note: Three values of parameter ‘i’ considered are 0.1, 0.6 and 0.9 representing minimal, intermediate and maximal contributions of innate preference, respectively. - Get the change in preference (ΔP) as the difference between P2 and P1.
- For estimating the variations arising due to selective and random demonstration, sort corresponding ΔP values according to the ascending order of P1 values. Following this proceed in following two manners:
- Select all the values of ΔP corresponding to P1 < 0.5. Plotting this subset of ΔP w.r.t P1 will give the distribution of N/2 preference values arising due to selective demonstration. This case is analogous to the scenario when demoFlavor is selectively chosen based on pre-STFP data.
- Select ΔP for every alternate P1 value resulting in total N/2 values with P1 ranging between 0 < P1 < 1. Plotting such ΔP values w.r.t P1 will give a distribution of N/2 preference values arising due to random demonstration. This case is analogous to the scenario when half the animals are randomly chosen for either demoFlavor without considering pre-STFP data.
- Compare statistical parameters of both ‘a’ and ‘b’ distributions to evaluate any differences in estimating ΔP.
- Select all the values of ΔP corresponding to P1 < 0.5. Plotting this subset of ΔP w.r.t P1 will give the distribution of N/2 preference values arising due to selective demonstration. This case is analogous to the scenario when demoFlavor is selectively chosen based on pre-STFP data.
- Repeat 1-7 for different standard deviations (SD2, SD3 etc.) in order to maximally cover the naturally occurring variability in SP and RP distributions for the population (Figure 5).
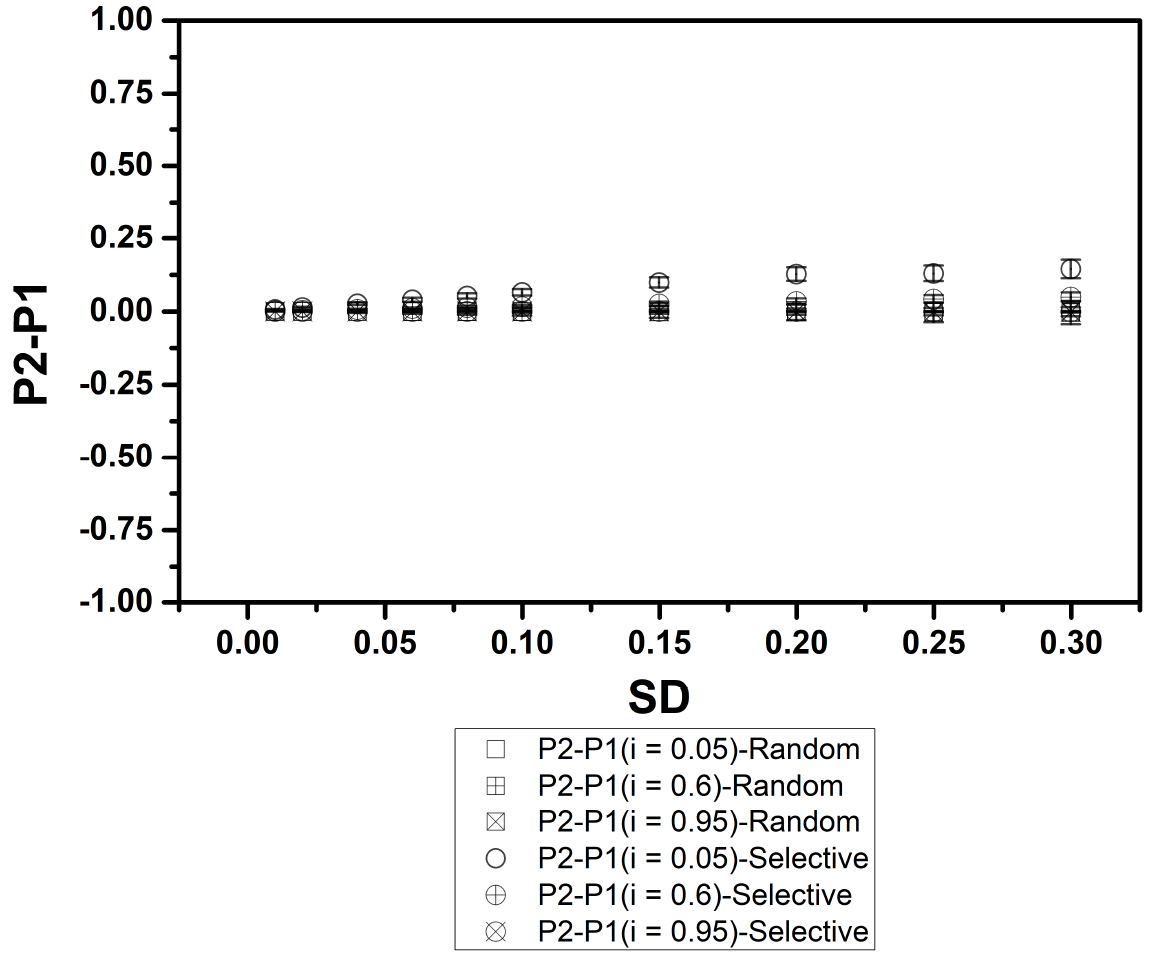
Figure 5. Change in preference (P2-P1 i.e., ΔP) plotted w.r.t the standard deviations (SD) for SP and RP distributions. Only for the scenario when contribution from innate preference is almost negligible (i = 0.05), we observe increasingly different ΔP selective demo (open circles) from ΔP random demo (Square) as population accommodates highly diverse preferences (increasing SD). For all other scenarios, specifically w.r.t cases having dominant contributions from innate preference (0.5 < i < 0.95), we observe that there is no difference between ΔP selective demo and ΔP random demo, even for diverse populations with higher SD.
- Generate first set of N random numbers with a mean of 0.5 and a fixed standard deviation (SD1). This set represents the distribution of ‘starting preference (SP)’ for N animals for demoFlavor. Each value represents SP for one virtual animal.
Recipes
- X% ‘Condiment’ flavored food
X g of ‘condiment’ powder mixed with (100-X) g of freshly ground food pellets
Note: We have predominantly used two flavor pairs, i) cocoa (2%) and cinnamon (1%) and, ii) thyme (1%) and basil (0.8%). For both mice groups, plain powdered pellets are mixed with respective flavor to make flavored food (for 2.0% cocoa flavored food, 2 g of powdered cocoa is mixed with 98 g of plain powdered pellets). We acquired commercially available condiments as flavoring agents (SNAPIN herbs and spices, Lotus household product, India). We have chosen the flavor concentrations based on previous studies (Holmes et al., 2002; Ross and Eichenbaum 2006; Smith et al., 2007; Lesburguères et al., 2011) and from pilot experiments conducted in our lab. - Cleaning solution
0.1% sodium hypochlorite solution in filtered tap water
Acknowledgments
This protocol has been adapted from a recent study published from our lab (Singh et al., 2017). This work was supported by Department of Science and Technology grants DSTO/BCN/BJ/1102 (Ramanujan Fellowship to JB) and DSTO/BCN/BJ/1297, Department of Biotechnology grants DBTO/BCN/BJ/0402 and DBT-IISc Partnership Program, Tata Trust grant JTT/MUM/INST/IIOS/201314/0033 and Council for Scientific and Industrial Research grants CSIR-09/079(2590)/2012-EMR-I (CSIR Fellowship to AS). The authors have no conflicts of interest.
References
- Choleris, E., Clipperton-Allen, A. E., Gray, D. G., Diaz-Gonzalez, S. and Welsman, R. G. (2011). Differential effects of dopamine receptor D1-type and D2-type antagonists and phase of the estrous cycle on social learning of food preferences, feeding, and social interactions in mice. Neuropsychopharmacology 36(8): 1689-1702.
- Choleris, E., Clipperton-Allen, A. E., Phan, A. and Kavaliers, M. (2009). Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol 30(4): 442-459.
- Clark, R. (2012). Socially transmitted food preference (STFP) task protocol. Bio Protoc e224.
- Clark, R. E., Broadbent, N. J., Zola, S. M. and Squire, L. R. (2002). Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. J Neurosci 22: 4663-4669.
- Fox, E. A. and Byerly, M. S. (2004). A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol Regul Integr Comp Physiol 286(6): R994-1004.
- Galef Jr, B. G. (1977). Social transmission of food preferences : An adaptation for weaning in rats. J Comp Physiol Psychol 91: 1136-1140.
- Galef, B. G., Jr., Mason, J. R., Preti, G. and Bean, N. J. (1988). Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol Behav 42(2): 119-124.
- Galef, B. G., Jr. and Whiskin, E. E. (1998). Limits on social influence on food choices of Norway rats. Anim Behav 56(4): 1015-1020.
- Holmes, A., Wrenn, C. C., Harris, A. P., Thayer, K. E. and Crawley, J. N. (2002). Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav 1(1): 55-69.
- Lesburguères, E., Gobbo, O. L., Alaux-Cantin, S., Hambucken, A., Trifilieff, P. and Bontempi, B. (2011). Early tagging of cortical networks is required for the formation of enduring associative memory. Science 331(6019): 924-928.
- Ross, R. S. and Eichenbaum, H. (2006). Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci 26(18): 4852-4859.
- Singh, A., Kumar, S., Singh, V. P., Das, A. and Balaji, J. (2017). Flavor dependent retention of remote food preference memory. Front Behav Neurosci 11: 7.
- Smith, C. A., Countryman, R. A., Sahuque, L. L. and Colombo, P. J. (2007). Time-courses of Fos expression in rat hippocampus and neocortex following acquisition and recall of a socially transmitted food preference. Neurobiol Learn Mem 88(1): 65-74.
- Wrenn, C. C., Harris, A. P., Saavedra, M. C. and Crawley, J. N. (2003). Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci 117(1): 21-31.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Singh, A. and Balaji, J. (2017). Sensitive Estimation of Flavor Preferences in STFP Using Cumulative Time Profiles. Bio-protocol 7(21): e2601. DOI: 10.21769/BioProtoc.2601.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










