- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Co-culture of Mesenchymal Stem Cells and Endothelial Colony Forming Cells
Published: Vol 7, Iss 20, Oct 20, 2017 DOI: 10.21769/BioProtoc.2587 Views: 15694
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Murine Osteoblast and Osteoclast Co-culture on Demineralized Bone Paper for Bone Remodeling
Seema Amin [...] Jungwoo Lee
Jun 5, 2025 2222 Views

Assessing Human Treg Suppression at Single-Cell Resolution Using Mass Cytometry
Jonas Nørskov Søndergaard [...] James B. Wing
Aug 20, 2025 2909 Views

Isolation and Co-culture of Paneth Cells and Intestinal Stem Cells
Ryosuke Isotani [...] Toshimasa Yamauchi
Sep 20, 2025 3460 Views
Abstract
The discovery of endothelial colony forming cells (ECFCs) with robust self-renewal and de novo vessel formation potentials suggests that ECFCs can be an excellent cell source for cardiovascular diseases treatment through improving neovascularization in the ischemic tissues. However, their engraftment after transplantation resulted to be low. Previous studies showed mesenchymal stem/stromal cells (MSCs) could improve the survival and capillary formation capacity of ECFCs in co-culture systems. In this article, we describe a protocol for in vitro co-culture of MSCs and ECFCs to prime ECFCs for better engraftment.
Keywords: Endothelial colony forming cellsBackground
Endothelial progenitor cells (EPC) are defined as a cell population capable of forming new blood vessels through a vasculogenesis process. In 2004, Ingram et al. identified a specific highly proliferative population of EPC in ex vivo culture termed ‘endothelial colony-forming cells (ECFC)’ from human umbilical cord blood (Ingram et al., 2004) and these cells have recently been declared to represent EPCs (Medina et al., 2017). A similar population can also be isolated from the human term placenta tissue with equivalent vascularization potential and at clinically relevant quantities (Patel et al., 2013; Shafiee et al., 2015). Therefore, ECFC transplantation has been proposed as a therapeutical approach for ischemic diseases such as myocardial infarction or critical leg ischemia. However, ECFCs engraftment and vasculogenic potential after transplantation are well documented to be low (Shafiee et al., 2017; Medina et al., 2017). Previous experiments have shown enhanced ECFC engraftment and function by co-transplantation of mesenchymal stem/stromal cells (MSC) with ECFC (Shafiee et al., 2017). In vitro and in the presence of MSC, ECFC showed enhanced survival in serum deprivation conditions. In normal/growth culture conditions, MSC co-culture resulted in reduced ECFC proliferation and altered appearance towards an elongated mesenchymal-like morphology. Further investigations suggested that direct contact with MSC was required for changes in ECFC morphology and proliferation rate (Shafiee et al., 2017). In addition, after being co-cultured with MSCs for 4 days, ‘primed ECFCs’ showed reduced colony forming potential but improved capacity to form tube-like structures on MatrigelTM in vitro (Shafiee et al., 2017). In this article, we describe a protocol for in vitro co-culturing of ECFCs and bone marrow-derived MSCs (BM-MSCs).
Materials and Reagents
- Materials
- 15 ml centrifuge tube (Corning, Falcon®, catalog number: 352196 )
- 50 ml centrifuge tube (Corning, Falcon®, catalog number: 352070 )
- T75 flasks
- 2 ml micro tubes
- Transwell chambers with a 0.4 µm pore size membrane (Corning, catalog number: 3397 )
- 0.22 μm filter (EMD Millipore, catalog number: SLGP033RS )
- Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-3 membrane (molecular weight cut off, 3 kDa) (EMD Millipore, catalog number: UFC900308 )
- NuncTM 6-well plate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 140675 )
- NuncTM 96-well flat bottom (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 167008 )
- 24-well plates (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 140644 )
- Culture slides (Corning, Falcon®, catalog number: 354118 )
- Cell strainer (size: 40 μm) (Corning, Falcon®, catalog number: 352340 )
- 20 µl, 100 µl, and 1 ml pipette tips
- 5 ml, 10 ml, and 50 ml serological pipettes
- Cryogenic vials, 1.2 ml (Corning, catalog number: 430487 )
- 50 ml syringe (BD, catalog number: 1018841 )
- Fluorescence activated cell sorting (FACS) tubes (5 ml Round-Bottom Polypropylene) (Corning, Falcon®, catalog number: 352063 )
- FACS tubes (5 ml Round-Bottom Polystyrene, with Cell Strainer Snap Cap) (Corning, Falcon®, catalog number: 352235 )
- 15 ml centrifuge tube (Corning, Falcon®, catalog number: 352196 )
- Cells
- Human MSCs: Adult BM-MSCs were purchased from Lonza (Lonza, USA)
- Human ECFCs: Human fetal placental ECFCs were isolated as reported previously (Patel et al., 2013). To distinguish between MSCs and ECFCs after co-culture, we suggest using GFP tagged ECFCs as used in the current protocol but non-GFP tagged cells can also be used
- Human MSCs: Adult BM-MSCs were purchased from Lonza (Lonza, USA)
- Reagents
- Double-distilled water (ddH2O)
- 100% ethanol
- Dulbecco modified Eagle medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 11995073 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437028 )
- Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
- PBS tablet (Sigma-Aldrich, catalog number: P4417 )
- TrypLE-express dissociation reagent (Thermo Fisher Scientific, GibcoTM, catalog number: 12605093 )
- Collagen, Type I solution from rat tail (concentrated stock, 100x) (Sigma-Aldrich, catalog number: C3867 )
- Acetic acid (Sigma-Aldrich, catalog number: ARK2183 )
- Endothelial basal medium-2 (EBM-2) (Lonza, catalog number: 190860 )
- Endothelial growth medium-2 (EGM-2) BulletKitTM (Lonza, catalog number: CC-3162 )
Note: The components of this kit includes the following items: Hydrocortisone, GA-1000 (Gentamicin, Amphotericin-B), hEGF, VEGF, hFGF-B, R3-IGF-1, Ascorbic acid, Heparin.
- Growth factor reduced MatrigelTM Matrix (Corning, catalog number: 356230 )
- Dimethyl sulfoxide (DMSO) (Fisher Scientific, catalog number: BP231-100 )
- Secondary antibody goat anti-rabbit Alexa Fluor® 488 conjugate (Thermo Fisher Scientific, InvitrogenTM, catalog number: A-11034 )
- Secondary antibody goat anti-mouse Alexa Fluor® 568 conjugate (Thermo Fisher Scientific, InvitrogenTM, catalog number: A-11004 )
- PE/Cy5 conjugated anti-human CD90 antibody (Thermo Fisher Scientific, eBioscienceTM, catalog number: 15-0909-42 )
- V450 mouse anti-human CD31 (BD, BD Biosciences, catalog number: 561653 )
- 7-Aminoactinomycin D (7-AAD) (Thermo Fisher Scientific, InvitrogenTM, catalog number: A1310 )
- Prolong Gold reagent with 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Thermo Fisher Scientific, catalog number: P36935 )
- Dulbecco’s phosphate buffered saline (D-PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190250 )
- EDTA (Merck, USA, CAS: 6381-92-6)
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2153 )
- Paraformaldehyde powder (PFA) (Sigma-Aldrich, catalog number: P6148 )
- 100% Triton X-100 (Fisher Scientific, catalog number: BP151-500 )
- Tween 20 (Sigma-Aldrich, catalog number: P9416 )
- Penicillin/streptomycin 10,000 U/ml (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Normal goat serum
- Trypan blue powder
- 70% ethanol (see Recipes)
- Phosphate buffered saline (PBS) (see Recipes)
- Coating solution (see Recipes)
- Complete EGM2 medium (see Recipes)
- Freezing medium (see Recipes)
- FACS buffer (see Recipes)
- 4% paraformaldehyde (PFA) (see Recipes)
- 0.1% Triton X-100 (see Recipes)
- Washing solution (see Recipes)
- Antibodies diluting solution (see Recipes)
- Blocking solution (see Recipes)
- 0.4% Trypan blue solution (see Recipes)
- Double-distilled water (ddH2O)
Equipment
- Water bath
- Refrigerator
- Portable Pipet-aid
- Centrifuge (Eppendorf, catalog number: 5810 R )
- Laminar flow work bench
- Centrifuge
- Shaker
- Tissue culture incubator set at 37 °C, 5% CO2 (Memmert GmbH & Co., Nurnberg, Germany)
- Hemocytometer (Hausser Scientific, catalog number: 3110 )
- IncuCyte Zoom (Essen BioScience)
- Zeiss Axio microscope (Carl Zeiss)
- Fluorescence-activated flow cytometry (FACS) Aria 11u system (BD Biosciences, FACSAriaTM)
- Gallios flow cytometer (Beckman Coulter, Fullerton, CA, USA)
Software
- Kaluza Flow Cytometry Analysis Software
- GraphPad Prism 7 software
- ZEN 2.3 (Blue edition, Carl Zeiss Microscopy GmbH)
- ImageJ (Fiji)
Procedure
- Cell culture and maintenance
To distinguish between MSCs and ECFCs after co-culture, ECFCs were GFP-tagged using manufacturer’s protocol (https://www.systembio.com/products/imaging-and-reporter-vectors/bioluminescent-imaging-vectors/bliv101-cmv-gfp-t2a-luciferase/ System Biosciences, Mountain View, CA, USA). Alternatively, ECFCs can be identified from MSCs through their high expression level of platelet endothelial cell adhesion molecule (PECAM-1) also known as CD31.- Preparation and application of coating solution
- Coat tissue culture flasks/plates with fresh collagen type-I coating solution (working concentration of 1x, see Recipes) for 3-4 h under a laminar flow hood at room temperature. It is recommended to prepare the coating solution fresh and decap the culture vessels during coating step for optimal performance.
- Then discard the coating medium thoroughly and gently wash the flasks/plates with PBS (see Recipes). Repeat washing step twice.
Note: The coated flasks/plates can be kept at 4 °C for maximum 1 month.
- In vitro culture and freezing of placental ECFCs and BM-MSCs
- Upon defrosting, culture ECFCs (about 6.0 x 103/cm2) and BM-MSCs (about 4.0 x 103/cm2) in EGM2 (see Recipes), and DMEM + 10% FBS respectively.
- Change media twice per week and split the culture 1:3 when cultures reach approximately 90% of confluency. Discard the medium and gently wash the flasks with PBS. For subculturing, add TrypLE-express and incubate for 3-5 min, and then inactivate with full culture medium. Spin down the cell suspension at 330 x g for 5 min and then discard the supernatant and resuspend in fresh media.
- For future applications freeze the cells using the freezing media (90% FBS and 10% DMSO, see Recipes).
- In vitro co-culture assays
- Direct co-culture of MSCs and ECFCs
To investigate the effects of MSCs co-culturing on ECFCs survival in stress conditions (here serum deprivation conditions), MSCs and ECFCs are co-cultured together in EBM-2 alone (Figure 1A). - Culture 2.5 x 105 MSCs and 2.5 x 105 ECFCs together in collagen coated T75 flasks with EGM2.
- After 4 h and once cells attached, discard the supernatant and wash the cells using EBM-2 (without addition of serum or growth factors) to completely remove any serum leftovers and feed the cells with EBM-2. As a control, 2.5 x 105 ECFCs are cultured in collagen-coated T75 flasks containing EBM-2.
- After 48 h, detach cells using TrypLE-express dissociation reagent and count them.
- Wash the cells with PBS, and resuspend them in FACS buffer (cell concentration: 1.0 x 105 to 5.0 x 105 in 100 µl FACS buffer, see Recipes) and transfer to 2 ml microtubes and then stain with mouse PE/Cy5 conjugated anti-human CD90 antibody (1:100 dilution), CD31 antibody (1:50 dilution) and 7-AAD (1:50 dilution) at 4 °C.
- After 20 min, add 1 ml FACS buffer to each microtube and centrifuge cells at 400 x g for 4 min and resuspend cells in 200 µl of FACS buffer.
- Use the Gallios flow cytometer and measure the percentage of ECFCs (GFP+CD31+CD90-) and calculate ECFCs number using appropriate flow cytometry analysis software (Kaluza).
- (Optional) To assess the impact of MSCs on ECFCs in normal culture conditions, in separate experiments culture 2.5 x 105 MSCs and 2.5 x 105 ECFCs together in collagen-coated T75 flasks containing EGM2 for 48 h, and calculate the ECFC number as before (Figure 1B).
Note: As a control, culture 2.5 x 105 ECFCs alone in collagen-coated T75 flasks containing EGM2 for 48 h.
- Indirect co-culture of Human Mesenchymal Stromal Cells (MSC) and ECFC
To assess the impact of cell-cell contact on ECFC survival, indirect co-culture experiment is performed using Transwell chambers with a 0.4-mm pore size membrane (Figure 1C). - Culture 0.5 x 105 MSCs on the top and 0.5 x 105 ECFCs in the bottom of Transwell chambers in separate plates with EGM2.
- After 4 h, transfer the upper compartment on the top of ECFC cultures wells and change the EGM2 with EBM-2 and incubate in normal culture conditions:
5% CO2
20% oxygen
37 °C
Humidity is maintained by putting a dish with sterile water
- After 48 h, remove the Transwell, detach ECFCs using TrypLE-express dissociation reagent and count the number of viable ECFCs by the trypan blue exclusion method using a hemocytometer.
Note: Use ECFCs on the bottom of Transwell chambers as control culture and leave the top compartment cell-free.
- Direct co-culture of MSCs and ECFCs
- Preparation and application of MSC-derived conditioned medium (MSC-CM)
To investigate the contribution of MSC-secreted factors on ECFCs, ECFCs are cultured in the presence of MSC-CM (Figure 1D).- Culture 5 x 105 MSCs in a T75 flask with EBM-2 and incubate in normal cell culture conditions.
- After 48 h, collect the supernatant and centrifuge at 330 x g for 5 min to remove any dead cells.
- Filter the supernatant through a 0.22 µm filter to remove any remaining cell debris. The MSC-CM can immediately be used.
- In the meantime, culture 5.0 x 105 ECFCs in EGM2 in a T75 tissue culture flask. After 4 h, remove the medium and wash the cultures with PBS and culture ECFCs with MSC-CM and incubate in normal culture conditions.
- After 48 h, remove the supernatant and then count the number of viable ECFCs by the trypan blue exclusion method using a hemocytometer. As control culture, use ECFCs in EBM-2 and EGM2.
Note: MSC-CM can be stored at -20 °C prior to use.
- The MSC-CM can be concentrated using a centricon column. Under the laminar hood assemble the Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-3 membrane column according to manufacturer’s instructions.
- Add 15 ml MSC-CM to the loading chamber (top) of the column. Centrifuge at 4 °C at 4,000 x g for 30 min. Concentrated MSC-CM can immediately be transferred on top of the ECFCs or can be stored at -20 °C after collection. 50 folds of concentrating are recommended.
- In the meantime, culture ECFCs in collagen-coated 6-well plates (5 x 104/well) with EGM2 and place in the incubator. After 4 h, remove the medium and wash the plates with PBS and deliver concentrated MSC-CM on top of ECFCs.
- After 48 h, count the number of viable ECFCs by the trypan blue exclusion method using a hemocytometer. As a control, culture ECFCs in EBM-2 and EGM2.
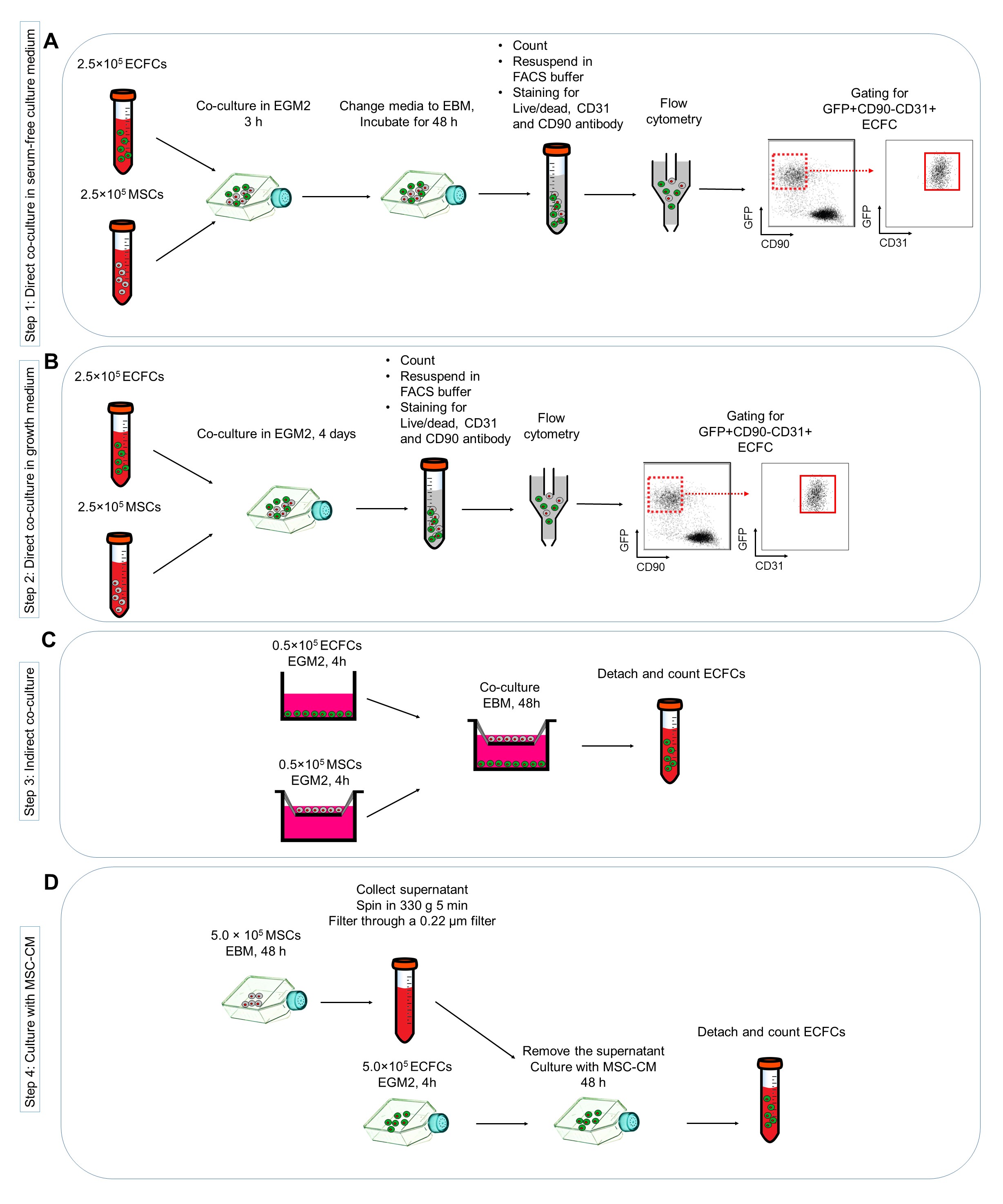
Figure 1. Protocols for investigating the effects of mesenchymal stem cells (MSC) co-cultured with endothelial colony forming cells (ECFC) in vitro. A. Direct co-culture of MSC and ECFC in serum-free culture medium; B. Direct co-culture in growth medium; C. Indirect co-culturing MSC and ECFC; D. Harvesting the MSC conditioned medium (MSC-CM) and deliver on ECFC cultures.
- Culture 5 x 105 MSCs in a T75 flask with EBM-2 and incubate in normal cell culture conditions.
- Immunostaining analysis
- Co-culture 5.0 x 103 of MSCs and 5.0 x 103 of ECFCs in collagen-coated culture slides with EGM2 and incubate in normal condition. Change the media every second day.
- After 4 days, discard the media, wash the cultures using DPBS and fix using 4% paraformaldehyde (PFA) solution (see Recipes) in PBS (the homemade PBS is used for immunostaining experiments) for 20 min at 4 °C.
Note: For CD31 immunostaining, either use 2% PFA for 15 min at 4 °C or apply freezer cold acetone for 10 min at 4 °C.
- Permeabilize cells with PBS/Triton X-100 (0.1%) (see Recipes) for 10 min at room temperature (RT).
Note: Excess incubation with PBS/Triton X-100 (0.1%) can damage cell morphology.
- To block nonspecific protein-binding sites, wash the slides with BSA blocking solution (see Recipes) for 20 min at RT.
- Then, incubate slides for 2 h at RT with primary antibodies against CD31 (1:50), CD144 (1:100) and CD90 (1:100) diluted in antibody diluting solution (PBS/BSA [3%]/Tween [0.1%], see Recipes).
- Wash the slides with 300 µl of PBS/Tween (0.1%) solution for 5 min. Repeat three times.
- Then, incubate slides for 45 min at RT with 250 µl secondary antibodies diluted in antibody diluting solution (PBS/BSA [3%]/Tween [0.1%]).
- Wash the slides with 300 µl of PBS/Tween (0.1%) solution for 5 min. Repeat three times.
- Take off the upper compartment of culture slide according to manufacturer’s instruction.
- Put one drop of Prolong Gold reagent with DAPI in each well of the 8-well plate, and cover the 8 wells with coverslip and observe under an Axio microscope.
- Co-culture 5.0 x 103 of MSCs and 5.0 x 103 of ECFCs in collagen-coated culture slides with EGM2 and incubate in normal condition. Change the media every second day.
- Preparation of primed-ECFCs
- Co-culture 2.5 x 105 of MSCs and 2.5 x 105 of ECFCs in collagen-coated T75 tissue culture flasks with EGM2 and incubate in normal condition. Change the media every second day (Figure 2A). Figure 3 shows representative images of ECFC alone or co-cultured with MSC in EGM2 for 4 days.

Figure 2. Preparation (A) and characterization (B) of primed-endothelial colony forming cells (ECFC). MSC: mesenchymal stem/stromal cells.
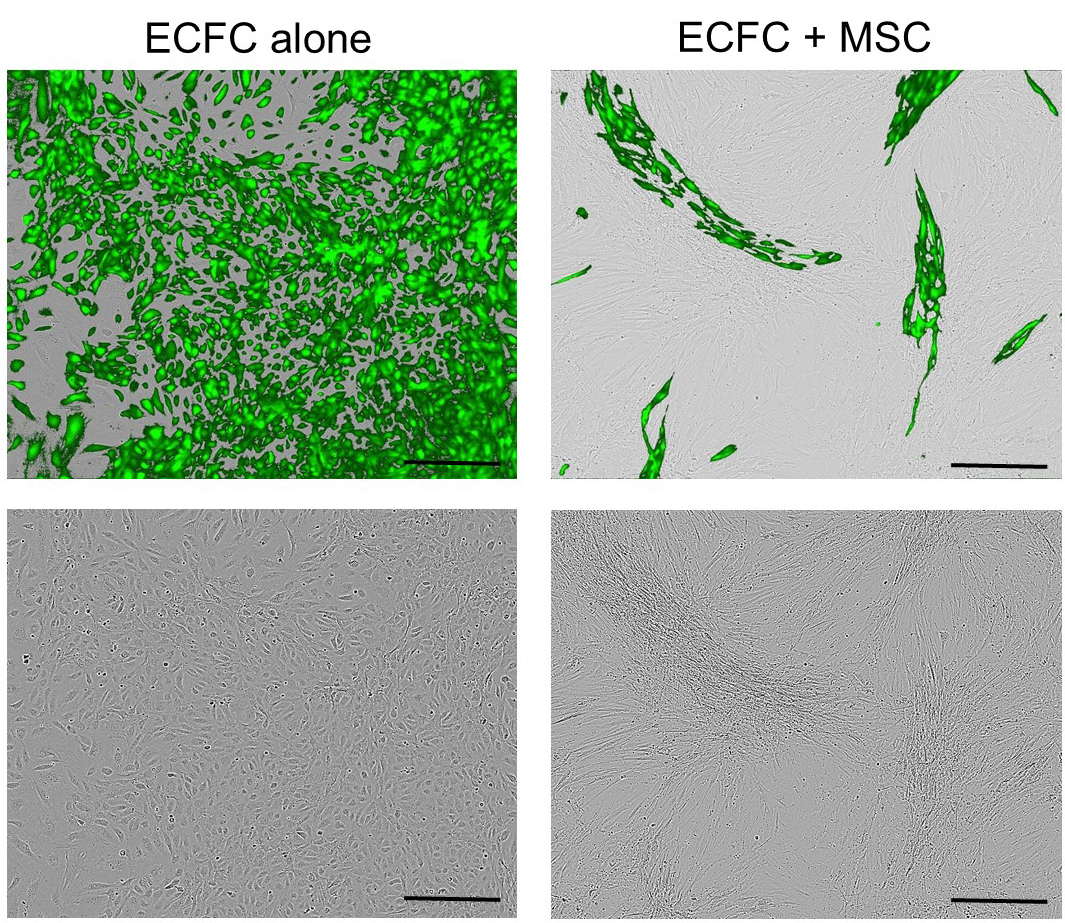
Figure 3. Representative images of GFP tagged-endothelial colony forming cells (ECFC) alone or co-cultured with mesenchymal stem/stromal cells (MSC) in EGM2 for 4 days. The top panels: merged phase contrast and fluorescent images; the down panels: phase contrast images. Scale bars = 100 µm.
- After 4 days discard the media, wash the cultures using DPBS and detach cells using TrypLE-express dissociation reagent and count them. Spin down cells at 330 x g for 5 min, and resuspend them in 100 µl ice-cold FACS buffer and stain with mouse PE/Cy5 conjugated anti-human CD90 antibody (1:100 dilution) and 7-Aminoactinomycin D (7-AAD, 1:50 dilution) and V450 conjugated anti-human CD31 antibody (1:50 dilution) at 4 °C.
- After 20 min, centrifuge cells at 330 x g for 4 min and resuspend cells in 100 µl of ice-cold FACS buffer.
Note: When performing flow cytometry, single stained samples are important to determine the levels of staining and compensation, so staining of MSCs alone for CD90 antibody, and ECFCs for CD31 is recommended. Dead cells can result in false positives results due to autofluorescence and increased level of non-specific binding. So, application of live/dead markers to eliminate dead cells is also recommended.
- Filter the cells through a 40 μm cell strainer to reduce cell aggregates before running samples through a FACS machine. Sort the GFP+CD90-CD31+ cells (primed-ECFCs) in ice-cold FBS in Falcon round-bottom polypropylene tubes.
Note: Sorted cells in PBS (or PBS with BSA/FBS) have limited viability, so sorting in FBS is recommended.
- These cells can be then subjected to tube-formation assay, limiting dilution assay and gene expression analysis.
- In separate experiments, and as control culture ECFCs alone in a T75 flask with EGM2 for 4 days and sort the GFP+CD90-CD31+ cells.
Note: Change the medium at day 2.
- Co-culture 2.5 x 105 of MSCs and 2.5 x 105 of ECFCs in collagen-coated T75 tissue culture flasks with EGM2 and incubate in normal condition. Change the media every second day (Figure 2A). Figure 3 shows representative images of ECFC alone or co-cultured with MSC in EGM2 for 4 days.
- Tube-formation assay
- Thaw MatrigelTM one night before use at 4 °C.
- Pre-chill the 24-well plates and the pipet tips at 4 °C for 2-3 h.
- Aliquot 100-150 μl of MatrigelTM per well of the pre-chilled 24-well plate on ice (Figure 2B).
Note: Make sure the MatrigelTM is evenly distributed over the wells and try to avoid air bubble formation inside the gel. MatrigelTM must not dry.
- Leave the coated plates in an incubator (humidified 37 °C incubator) and allow gel to polymerize for 30 to 60 min.
- Transfer the ECFCs harvested from sort (primed-ECFCs) to 15 ml tubes and spin down at 330 x g for 5 min. Remove the supernatant and resuspend cells in fresh EGM2. Count the cells and dispense 400 μl of primed-ECFCs (1.0 x 104) into the MatrigelTM-coated wells. Use 1.0 x 104 non-primed ECFCs as a control.
- Place the plates in the IncuCyte® live-cell analysis system, and capture phase contrast and fluorescence images every 2 h for 48 h. Count capillary-like structures. Figure 4 shows representative images of primed ECFCs seeded on MatrigelTM.
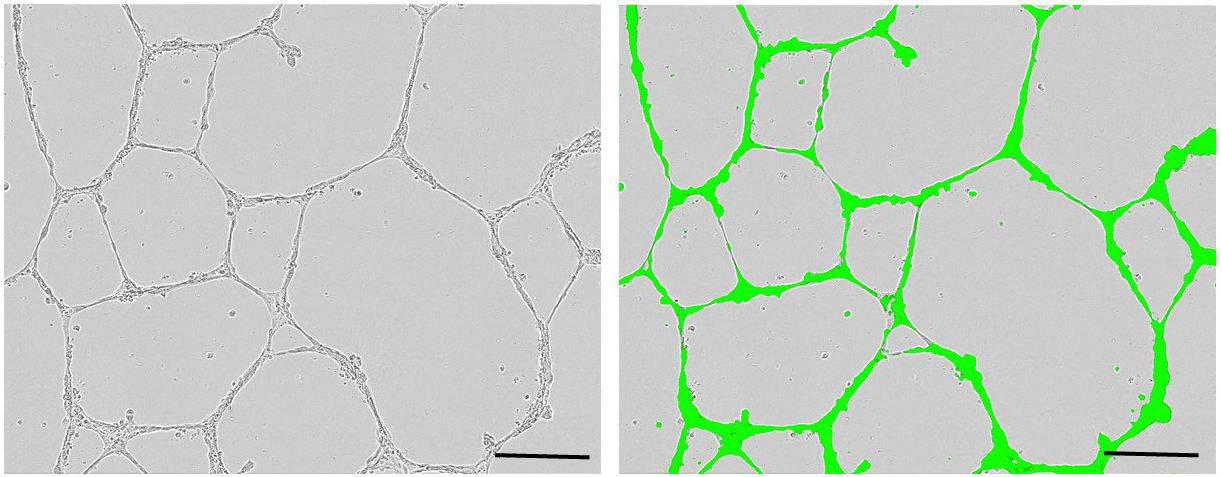
Figure 4. Representative images of primed-endothelial colony forming cells seeded on MatrigelTM after 24 h (left: phase contrast, right: phase contrast merged with fluorescent). Scale bars = 100 µm.
- Thaw MatrigelTM one night before use at 4 °C.
- Single cell assay
- Coat desired number of 96-well plate with 50 μl coating solution for 3-4 h under the laminar flow hood and make sure the coating solution is evenly distributed over the wells (Figure 2B). It is recommended to prepare the coating solution fresh and decap the plates during coating step for optimal performance.
- Discard the coating medium thoroughly and gently wash the flasks with PBS. Repeat washing step twice.
Note: The coated 96-well plate can be kept at 4 °C for maximum 1 month.
- Prior to cell culture, aliquot 100-150 μl of EGM2 into each well of 96-well plate.
- Seed one single primed or non-primed ECFCs (GFP+CD90-CD31+) in each well by using the BD FACSAria sort machine. Incubate plates in normal cell culture conditions and change the media with fresh EGM2 twice a week by removing half of the medium and replacing it with fresh EGM2.
- After 2 weeks, visualize the cells under a light microscope and count/score colonies. Endothelial colonies can be scored as endothelial clusters (ECs; < 50 cells), low proliferative potential EPCs (LPP; 50-2,000 cells), and high proliferative potential EPCs (HPP; > 2,000 cells) (Figure 5).
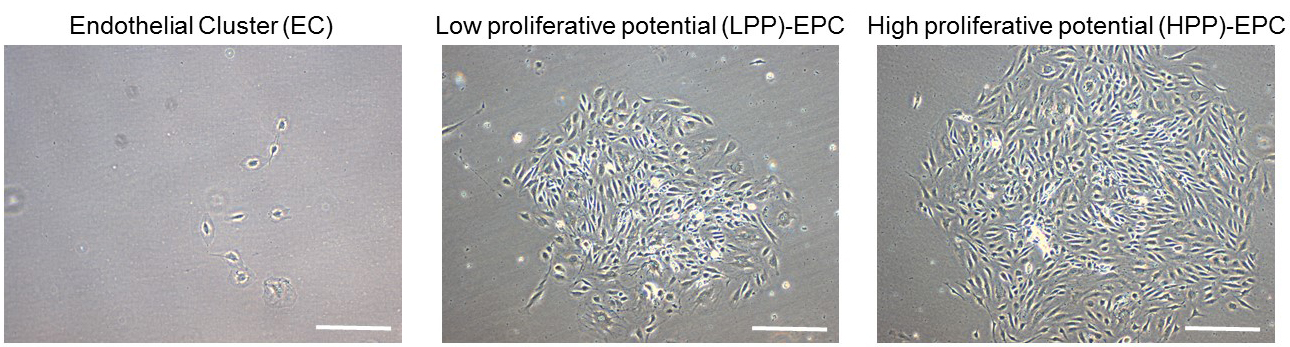
Figure 5. Representative images of primed-endothelial colony forming cells subjected to single cell assay after 10 days. Endothelial colonies can be scored as endothelial clusters (EC), low proliferative potential endothelial progenitor cells (EPCs) (LPP), and high proliferative potential EPCs (HPP). Scale bars = 100 µm.
- Coat desired number of 96-well plate with 50 μl coating solution for 3-4 h under the laminar flow hood and make sure the coating solution is evenly distributed over the wells (Figure 2B). It is recommended to prepare the coating solution fresh and decap the plates during coating step for optimal performance.
Data analysis
- Kaluza software can be used to analyze the flow data. For direct and indirect co-culture assays at least six biological replicates should be used. Limiting dilution and tube-formation assays are performed in triplicate and quadruplicate respectively for at least 3 independent experiments. For the tube-formation assay, we counted capillary-like structures in at least 20 fields of view.
- All Statistical analysis can be performed using GraphPad Prism 7 software. Paired or unpaired Student’s t-test can be used to determine statistical differences among different groups.
Notes
- For better functionality and reproducibility of the results, we recommend using stem/progenitor cells at passages 3-5 for all the experiments.
- FACS solutions, culture media, immunostaining solutions need to be prepared on the day of use for best results.
- All cell culture reagents should be warmed at 37 °C before use.
- For highest viability during the FACS sorting, cells should be kept on ice.
- We recommend to keep collagen type-I solution at 2-8 °C. After coating, completely remove coating solution by aspirating, then gently wash with PBS and keep at 2-8 °C before use. The coated flasks/plates should be stored at 2-8 °C. We recommend using the coated flask within 1 month.
Recipes
- 70% ethanol (1 L)
700 ml 100% ethanol
300 ml deionized water
- Phosphate buffer saline (PBS, 1 L)
Note: This is homemade PBS and only used for immunostaining experiments.
10 PBS tablets
1,000 ml MilliQ water
- Collagen coating solution (100 ml)
1,000 µl collagen solution (concentrated stock, 100x)
560 µl glacial acetic acid
99 ml sterile PBS
- Complete EGM2 medium (EBMTM-2 plus SingleQuotsTM of Growth Supplements) (500 ml)
500 ml EBMTM-2
10 ml fetal bovine serum (FBS)
0.5 ml hEGF
0.2 ml hydrocortisone
0.5 ml GA-1000 (Gentamicin, Amphotericin-B)
0.5 ml VEGF
2.0 ml hFGF-B
0.5 ml R3-IGF-1
0.5 ml ascorbic acid
0.5 ml Heparin
- Freezing medium
90% FBS
10% DMSO
- FACS buffer
1x D-PBS
2.5 mM EDTA
0.5% BSA
- 4% paraformaldehyde (PFA) (1 L)
40 g of paraformaldehyde powder
1,000 ml PBS
- 0.1% Triton X-100 (1 L)
1 ml of Triton solution
1,000 ml PBS
- Washing solution for immunofluorescent staining (1 L)
1 ml Tween 20
1,000 ml PBS
- Antibodies diluting solution for immunofluorescent staining
DPBS, pH 7.2-7.4
3% BSA
0.05% Tween 20
- Blocking solution for immunofluorescent staining (100 ml)
20 ml of normal goat serum
80 ml immunofluorescent washing solution
- 0.4% trypan blue solution (RT)
4 mg of trypan blue powder
1 ml of 1x PBS
Note: For counting the cells, dilute the cell suspension in a 1:1 dilution with 0.4% trypan blue solution and incubate for 1-2 min.
Acknowledgments
This protocol has been adapted and slightly modified from the previously published article (Shafiee et al., 2017). This study was supported by National Health and Medical Research Council Project Grant.
References
- Ingram, D. A., Mead, L. E., Tanaka, H., Meade, V., Fenoglio, A., Mortell, K., Pollok, K., Ferkowicz, M. J., Gilley, D. and Yoder, M. C. (2004). Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104(9): 2752-2760.
- Medina, R. J., Barber, C. L., Sabatier, F., Dignat-George, F., Melero-Martin, J. M., Khosrotehrani, K., Ohneda, O., Randi, A. M., Chan, J. K. Y., Yamaguchi, T., Van Hinsbergh, V. W. M., Yoder, M. C. and Stitt, A. W. (2017). Endothelial progenitors: A consensus statement on nomenclature. Stem Cells Transl Med 6(5): 1316-1320.
- Patel, J., Seppanen, E., Chong, M. S., Yeo, J. S., Teo, E. Y., Chan, J. K., Fisk, N. M. and Khosrotehrani, K. (2013). Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Transl Med 2(11): 839-847.
- Shafiee, A., Fisk, N. M., Hutmacher, D. W., Khosrotehrani, K. and Patel, J. (2015). Fetal endothelial and mesenchymal progenitors from the human term placenta: potency and clinical potential. Stem Cells Transl Med 4(5): 419-423.
- Shafiee, A., Patel, J., Wong, H. Y., Donovan, P., Hutmacher, D. W., Fisk, N. M. and Khosrotehrani, K. (2017). Priming of endothelial colony-forming cells in a mesenchymal niche improves engraftment and vasculogenic potential by initiating mesenchymal transition orchestrated by NOTCH signaling. FASEB J 31(2): 610-624.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Shafiee, A. and Khosrotehrani, K. (2017). In vitro Co-culture of Mesenchymal Stem Cells and Endothelial Colony Forming Cells. Bio-protocol 7(20): e2587. DOI: 10.21769/BioProtoc.2587.
Category
Stem Cell > Adult stem cell > Endothelial stem/progenitor cell
Developmental Biology > Cell growth and fate > Angiogenesis
Cell Biology > Cell isolation and culture > Co-culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









