- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Establishing a Symbiotic Interface between Cultured Ectomycorrhizal Fungi and Plants to Follow Fungal Phosphate Metabolism
Published: Vol 7, Iss 20, Oct 20, 2017 DOI: 10.21769/BioProtoc.2577 Views: 8680
Reviewed by: Scott A M McAdamZhaohui LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2634 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1568 Views

CAPS-Based SNP Genotyping for Nitrogen-Response Phenotypes in Maize Hybrids
Jannis Jacobs [...] Peter K. Lundquist
Dec 20, 2025 543 Views
Abstract
In ectomycorrhizal plants, the fungal cells colonize the roots of their host plant to create new organs called ectomycorrhizae. In these new organs, the fungal cells colonize the walls of the cortical cells, bathing in the same apoplasm as the plant cells in a space named the ‘Hartig net’, where exchanges between the two partners take place. Finally, the efficiency of ectomycorrhizal fungi to improve the phosphorus nutrition of their host plants will depend on the regulation of phosphate transfer from the fungal cells to plant cells in the Hartig net through as yet unknown mechanisms. In order to investigate these mechanisms, we developed an in vitro experimental device mimicking the common apoplasm of the ectomycorrhizae (the Hartig net) to study the phosphorus metabolism in the ectomycorrhizal fungus Hebeloma cylindrosporum when the fungal cells are associated or not with the plant cells of the host plant Pinus pinaster. This device can be used to monitor 32Phosphate efflux from the fungus previously incubated with 32P-orthophosphate.
Keywords: In vitro symbiotic interfaceBackground
The association between mycorrhizal fungi and plants is known to improve plant P nutrition (reviewed by Smith and Read, 2008; Plassard and Dell, 2010; Cairney, 2011; Smith et al., 2015). This positive effect is due to P uptake and P transport through the fungal cells exploring soil far away from the roots. The capacity of the fungus to take up P from the soil solution and to release P to mycorrhizal roots is therefore an important feature for its positive effect on plant P nutrition. In ectomycorrhizal symbiosis, we know (i) that the exchanges between the fungus and the plant occur in the Hartig net, located inside the ectomycorrhizae, and (ii) that there is no direct cellular connection via, for example plasmodesmata, between the plasma membrane of the fungal and the plant cells. Therefore, these exchanges are very difficult to study as they occur in the apoplasmic space of the Hartic net. Here, we describe an in vitro system enabling us to mimick this apoplasmic space for the ectomycorrhizal fungus Hebeloma cylindrosporum incubated with its host plant Pinus pinaster (Torres-Aquino et al., 2017). This method could be used with other fungal or plant species.
Materials and Reagents
- Sterile plastic 90 mm Petri dishes (Dominique DUTSCHER, Gosselin, catalog number: 688302 )
- Gloves, EcoSHIELD Natural Nitrile PF 250 (Dominique DUTSCHER)
- Multi-Purpose Silicone for kitchen or bathroom, 280 ml (Castorama, Rubson)
- PTFE (Polytetrafluoroethylene) microtube, 1.15 mm and 1.75 mm for internal and external diameter, respectively (Dominique DUTSCHER, PTFE, catalog number: 091932 )
- Filter paper without ash, grade 542, 185 mm diameter (Dominique DUTSCHER, WhatmanTM, catalog number: 1542185 )
- Autoclavable bags Polypropylene bag, 3 L, non-printed (Dominique DUTSCHER, catalog number: 140230 )
- Autoclave tape (Dominique DUTSCHER, catalog number: 490009 )
- Sterile 60 ml syringes 3 pieces (Dominique DUTSCHER, Omnifix, catalog number: 921010 )
- Sealing film for manual application, roll of 38 m x 100 mm (VWR, PARAFILM® M, catalog number: 291-1213 )
- Needles 18 G 0.9 x 40 mm (Dominique DUTSCHER, MicrolanceTM 3, catalog number: 301300 )
- Tubing, int diam 1.14 mm (Dominique DUTSCHER, Silicone, catalog number: 4906591 )
- Tubing, int diam 3.17 mm (Dominique DUTSCHER, Silicone, catalog number: 4906600 )
- 125 ml sterile polypropylene containers, red cap (Dominique DUTSCHER, catalog number: 688270 )
- Tips 200 µl for pipet (Dominique DUTSCHER, Sartorius, catalog number: 070468 )
- Tips 10 µl for pipet (Dominique DUTSCHER, Sartorius, catalog number: 077179 )
- Home-made needle holder for aeration
- Sterile syringe filters for air, 0.2 µm, 6.4 cm diam (Labomoderne, Midisart, catalog number: RS3320 )
- Nichrome wire, stainless steel, round, 22 gauge, 0.64 mm diameter (suppliers for electronic cigarettes)
- Valve luer polycarbonate one way (Cole-Parmer, catalog number: EW-30600-01 )
- Folding skirted caps, 14.9 mm diam (Dominique DUTSCHER, catalog number: 110603 )
- Paper for sterilisation (Dominique DUTSCHER, catalog number: 006950 )
- Pinus pinaster (maritime pine), seeds (Vilmorin, catalog number: PPA301 massif landais)
- Hebeloma cylindrosporum (ectomycorrhizal basidiomycete) (laboratory’s own collection, available upon request)
- Agar-agar (Sigma-Aldrich, catalog number: A7002-500G )
- D-glucose (Sigma-Aldrich, catalog number: G8270-1KG )
- Concentrated (30%) hydrogen peroxide (H2O2) solution (Sigma-Aldrich, catalog number: 216763-500ML-M )
- Manganese(II) sulfate monohydrate (MnSO4·H2O) (Sigma-Aldrich, catalog number: M7899-500G )
- Zinc sulfate heptahydrate (ZnSO4·7H2O) (Sigma-Aldrich, catalog number: Z0251-100G )
- Boric acid (H3BO3) (Sigma-Aldrich, catalog number: B6768-500G )
- Copper(II) sulfate pentahydrate (CuSO4·5H2O) (Sigma-Aldrich, catalog number: C8027-500G )
- Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) (Sigma-Aldrich, catalog number: C2786-500G )
- Potassium nitrate (KNO3) (Sigma-Aldrich, catalog number: P8291-500G )
- Potassium dihydrogen phosphate (KH2PO4) (EMD Millipore, catalog number: 105108 )
- Magnesium sulfate heptahydrate (MgSO4·7H2O) (Sigma-Aldrich, catalog number: 63138-250G )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333-500G )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C5080-500G )
- Calcium hydroxide (Ca(OH)2) (EMD Millipore, catalog number: 102047 )
- Ammonium iron(III) citrate (NH4FeC6H5O7) (Sigma-Aldrich, catalog number: RES20400-A702X )
- Calcium sulfate dihydrate (CaSO4·2H2O) (EMD Millipore, catalog number: 102161 )
- 2-N-morpholino-ethanesulfonic acid, 4-morpholineethanesulfonic acid monohydrate (MES) (Sigma-Aldrich, catalog number: 69892-500G )
- Tris(hydroxymethyl)aminomethane (TRIS) (Sigma-Aldrich, catalog number: T1378-500G )
- 1 N sulfuric acid solution (EMD Millipore, catalog number: 1.09072.1000 )
- Agar plates for germination (see Recipes)
- 0.1 N Ca(OH)2 solution(see Recipes)
- Trace elements (1,000 ml) (see Recipes)
- Mineral salt base solutions (100 ml) (see Recipes)
- N1 + P solution (1,000 ml) (see Recipes)
- CaSO4 solution (0.2 mM) (see Recipes)
- Interaction medium (1,000 ml) (see Recipes)
Equipment
- A pair of stainless steel straight tweezers Wironit, Brucelles type, 130 mm (Dominique DUTSCHER, catalog number: 491037 )
- Glass bottles, 250 ml, ISO borosilicate, graduated (Dominique DUTSCHER, catalog number: 046413B )
- Glass bottles, 1000 ml, ISO borosilicate, graduated (Dominique DUTSCHER, catalog number: 046415 )
- Glass bottles, 2,000 ml, ISO borosilicate, graduated (Dominique DUTSCHER, catalog number: 046416 )
- Borosilicate glass funnel, 60° angle, 8 ml (Dominique DUTSCHER, catalog number: 068957 )
- A standalone burner (Dominique DUTSCHER, catalog number: 071109 )
- Butane gas cartridge for the burner (Dominique DUTSCHER, catalog number: 060415 )
- Automatic Piezo electronic gas lighter (Dominique DUTSCHER, catalog number: 076275 )
- Air-pumps for aquarium (SuperFish, model: air-box Nr.4 )
- Graduated borosilicate glass beaker 400 ml (Dominique DUTSCHER, catalog number: 068939 )
- Graduated borosilicate glass beaker 1,000 ml (Dominique DUTSCHER, catalog number: 068942 )
- Polypropylene economical beaker 3,000 ml with moulded graduations (Dominique DUTSCHER, catalog number: 391134 )
- Soda-lime glass Petri dish 150 x 25 mm (Dominique DUTSCHER, catalog number: 068522 )
- Glass tubing, length 150 mm, ext diameter 8 mm (VWR, AR-Glas®, catalog number: SCOR1193467 )
- Test tube, borosilicate glass, height 150 mm, internal diameter 20 mm (VWR, Duran, catalog number: 212-1120 )
- Stainless steel racks for 6 x 4 tubes of 16-20 mm diameter (Dominique DUTSCHER, catalog number: 854061 )
- A nail and cutting pliers
- A glass cutting knife (Sigma-Aldrich, catalog number: Z136441 )
- One stainless steel spatula, 235 mm (Dominique DUTSCHER, catalog number: 001809 )
- Autoclave
- Laminar flow cabinet, horizontal (Dominique DUTSCHER, catalog number: 486084 )
- Growth cabinet with controlled light, temperature and humidity (Binder, model: KBF P 720 )
Software
- Microsoft Excel for calculations
- Statistica 7.1 (StatSoft Inc., Tulsa, OK, USA)
Procedure
- Preparation of equipment
- Test tubes for plant (based on those given in Plassard et al., 1994)
- Cut with a glass knife small pieces (around 5 cm long) from a glass tubing (ext diameter 8 mm, equipment #14 in the Equipment section), hold the cut piece with a pair of tweezers and polish each side over a flame.
- Glue one piece of cut glass tubing inside a test tube (equipment #15 in the Equipment section), with silicone paste as shown in Figure 1. Place the tubes on a rack and leave them to dry for 24 h.
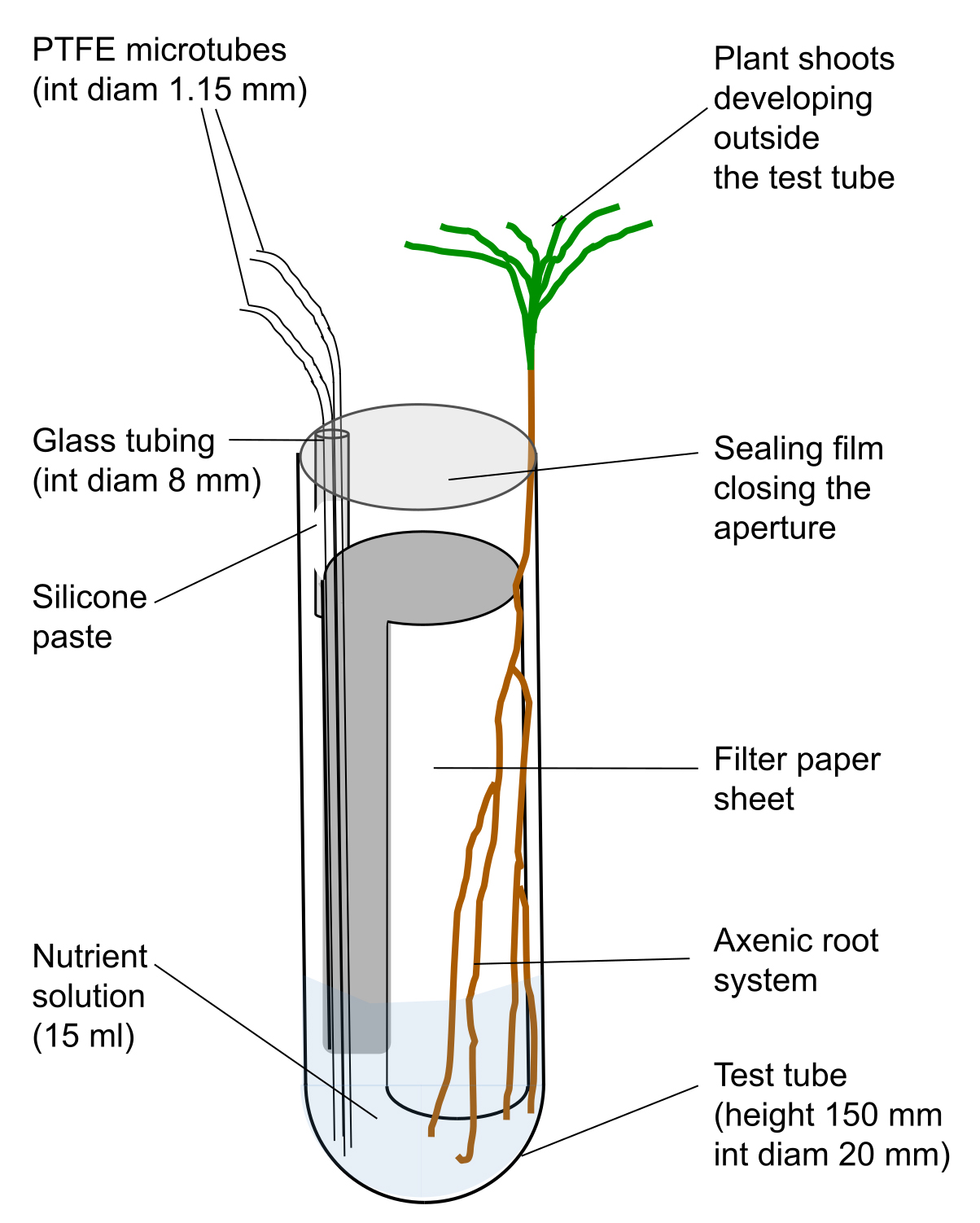
Figure 1. Scheme of test tube used to grow young Pinus pinaster seedlings
- Cut the PTFE microtube (material #4 in Materials and Reagents section) in pieces of 25 cm and 30 cm long. Place one piece of each length inside the glass tubing and fill the space between the glass and the microtube with silicone paste. The long microtube will be used to supply nutrient solution whereas the short one could be used to withdraw the solution or to supply aeration. Leave to dry for 24 h.
- Cut small pieces (20 mm) of silicone tubing (internal diameter 1.14 mm, material #11 from the Materials and Reagents section) and place it over the end of a PTFE microtube. Close the extremity of a 200 µl tip by passing it through a flame. After cooling, insert the burned extremity into the second opening of the silicone tubing to obtain a stopper to close the microtube.
- Cut pieces of filter paper Whatman of 34 x 125 mm and place one per tube.
- Add 10 ml of glucose solution in each tube. This volume is enough to saturate the filter paper.
- Place the rack with tubes in an autoclavable bag. Close it with tape and put another autoclavable bag. Close it with tape and sterilise the whole at 115 °C for 40 min. Repeat the sterilization after 48 h. Glucose will promote the germination of spores of unwanted saprophytic fungal species belonging to the genus Penicillium, for example, that will be killed by the second sterilization. The tubes are ready to receive a germinated seed.
- Cut with a glass knife small pieces (around 5 cm long) from a glass tubing (ext diameter 8 mm, equipment #14 in the Equipment section), hold the cut piece with a pair of tweezers and polish each side over a flame.
- Home-made syringe holders (Figure 2)
Cut the different pieces of wood according to the dimensions given in Figure 2. For example, to hold 6 syringes, the top piece is 37 cm long x 6 cm wide and the one underneath is 32 cm long x 6 cm wide. Adjust and maintain the two pieces together to drill the holes in a two-by-two alignment. Afterwards, assemble all the pieces together as shown in Figure 2.
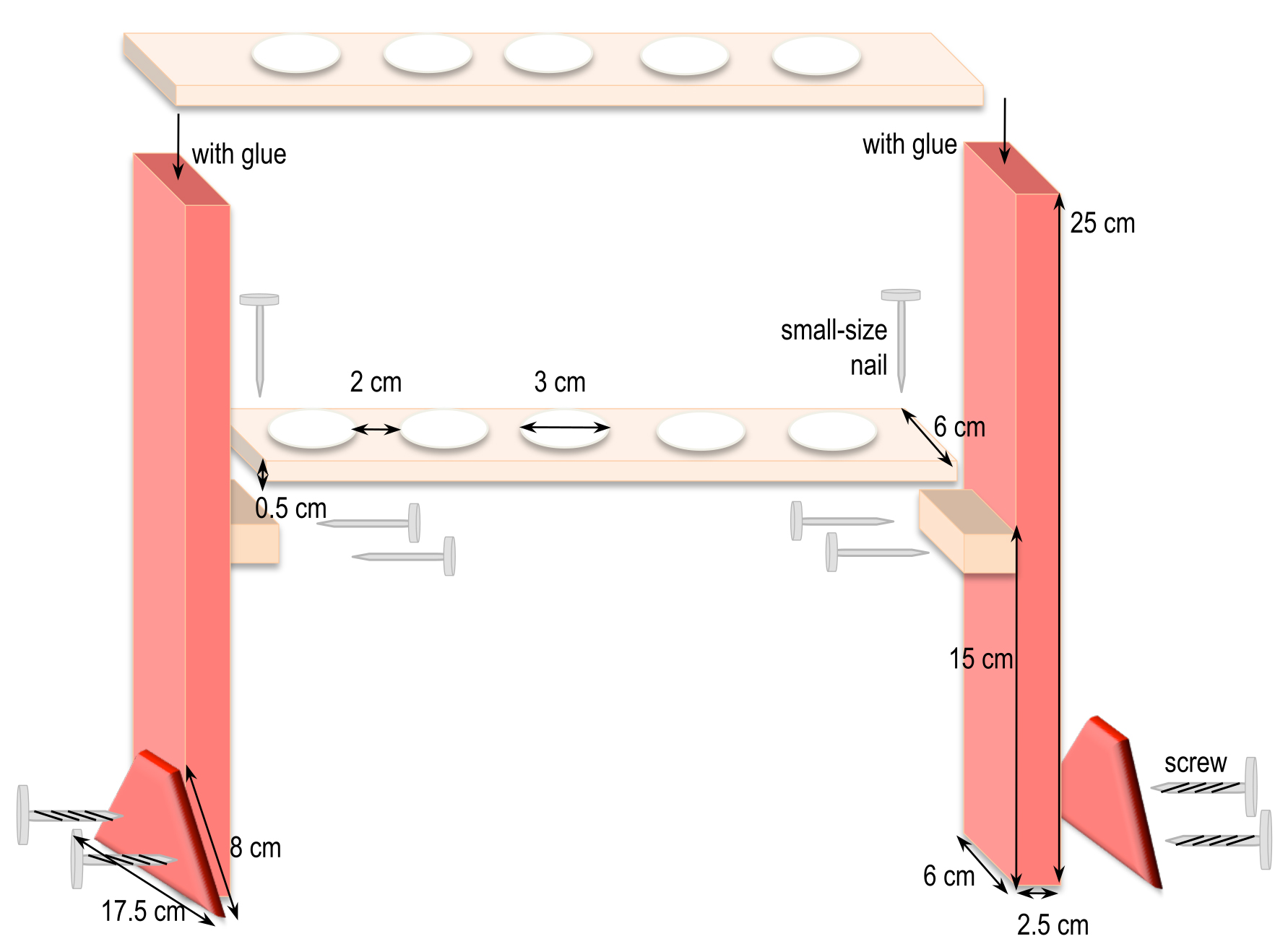
Figure 2. Homemade holders to support syringes for incubation of mycelia in interaction medium
- Preparation of connectors for aeration (Figure 3)
First, take an 18 G needle with a pair of tweezers, heat its collar over a flame to soften the glue and pull it with another pair of clamps. After cooling, adjust a small piece of silicone tube (1.14 mm internal diameter) of about 3 cm long on the top of the needle. Take a skirt cap and stitch 6 needles into its top and close its bottom by a 1 ml pipette tip previously cut at its finest end. If necessary, add silicone paste to fragile places to strengthen the airproof of the system. To the cut end of the blue pipette tip, add a piece of silicone tube (3.17 mm internal diameter) about 20 cm long that will be plugged later into a sterile air filter placed between the air pump and the connector (see Figure 4). Finally, place the whole system [connector + PTFE tubes + large diameter silicone tube] in an autoclavable bag and sterilize it by autoclaving (115 °C, 40 min).
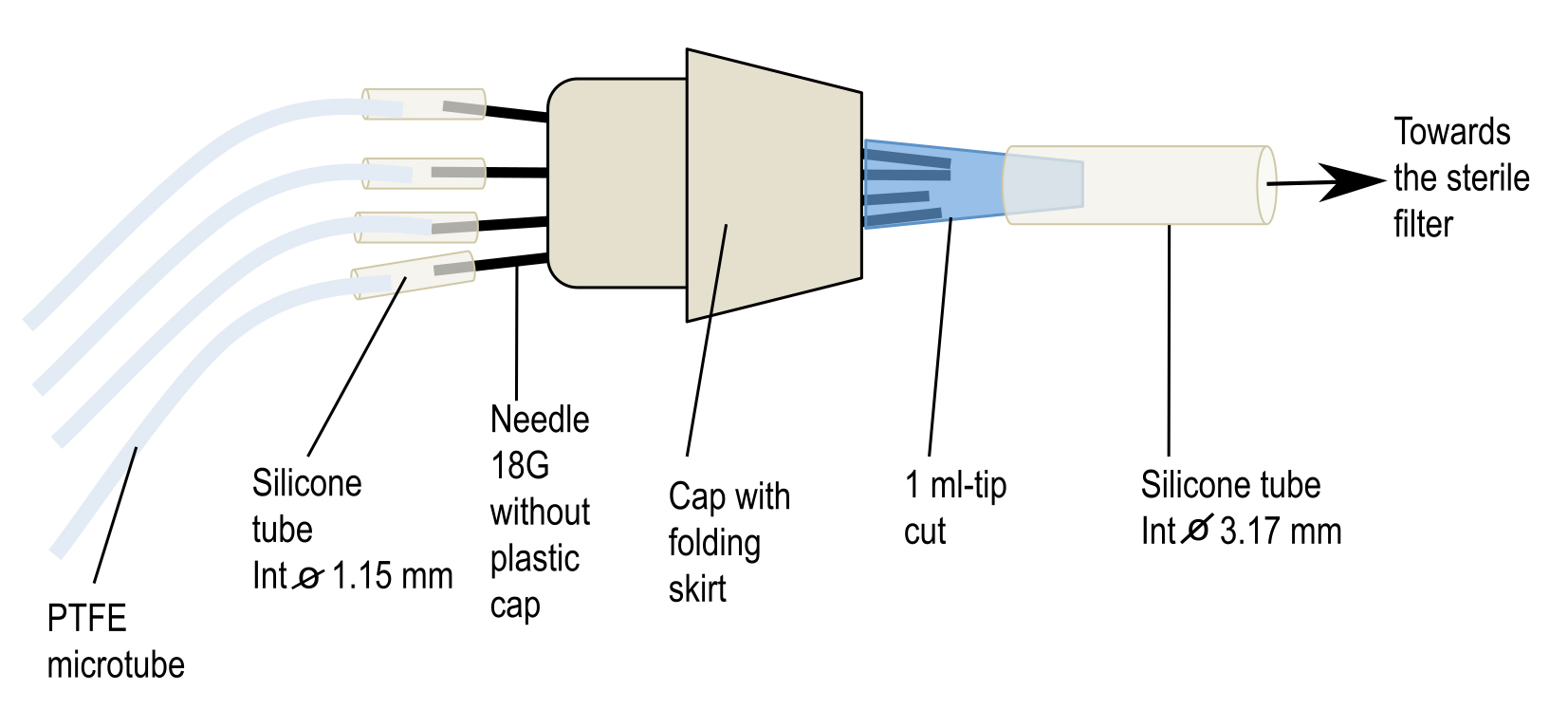
Figure 3. Homemade connector to aerate syringes during the incubation of mycelia in interaction medium
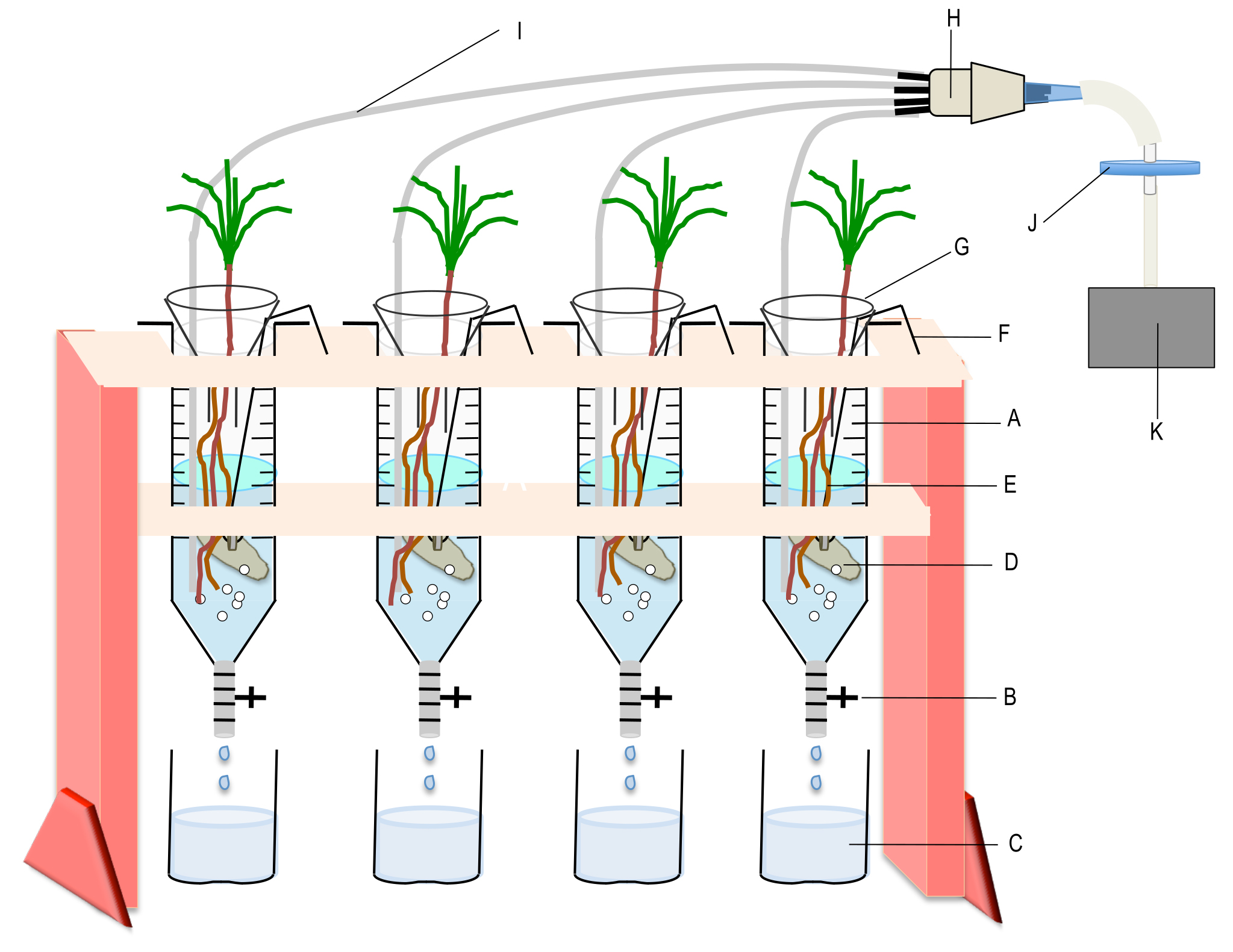
Figure 4. Device used to incubate the plants with the mycelia of the ectomycorrhizal fungus. A. Syringe containing the interaction medium; B. Polycarbonate valve; C. Collection vessel; D. Mycelium; E. Root system of the interacting plant; F. Nichrome wire bearing the mycelium; G. Glass funnel to minimize evaporation; H. Home-made connector for gathering 4 to 7 PTFE microtubes; I. PTFE microtube for individual aeration; J. Sterile filter; K. Air pump. Each syringe can contain up to three P. pinaster seedlings.
- Preparation of syringes for incubation of the fungus, with or without the plant:
Remove the plunger of each syringe and discard it. Using a hot nail, drill a hole at the top of the syringe opposite to the graduations giving the volume. This hole will be used to place the microtube for aeration inside the syringe. Place all drilled syringes in an autoclavable bag and sterilize it by autoclaving (115 °C, 40 min).
- Test tubes for plant (based on those given in Plassard et al., 1994)
- Seed disinfection and germination
- First, prepare germination plates by pouring 25 ml of Agar-agar + glucose (see Recipes) solution in 90 mm Petri dishes. Generally, 8 seeds are put in one Petri dish and this ratio is used to calculate the total number of Petri dishes to be prepared. In our work we use seeds of maritime pine (Pinus pinaster Soland in Ait.) of Médoc provenance, originating from Landes-Sore-VG (France). The seeds, whose number is converted in g using the weight of 10 seeds, are first soaked for 48 h at 4 °C in deionized water as we noticed that this soaking improved the success of disinfection.
- After removing water, the seeds are placed into a large Petri dish (diameter 150 mm) in a laminar flow cabinet. They are then covered with pure, concentrated H2O2 (30%). After 50 min, H2O2 solution is discarded. Then, the seeds are rinsed several times with roughly 1 L of sterile deionized water.
- After the last rinse, all the water is withdrawn from the dish with a sterile syringe and the seeds are allowed to become completely dry in the laminar flow cabinet. The seeds are then oriented with the root down and deposited on the solid medium, on a single line in the middle of the dish. The dish is closed with sealing film and placed vertically in a box to save space and to get straight roots that do not penetrate into the solid medium. The box is incubated in the dark, at 25 °C. The first seeds (about 10%) germinate after 10 days and the maximum of germination occurs within 2 to 3 weeks.
- First, prepare germination plates by pouring 25 ml of Agar-agar + glucose (see Recipes) solution in 90 mm Petri dishes. Generally, 8 seeds are put in one Petri dish and this ratio is used to calculate the total number of Petri dishes to be prepared. In our work we use seeds of maritime pine (Pinus pinaster Soland in Ait.) of Médoc provenance, originating from Landes-Sore-VG (France). The seeds, whose number is converted in g using the weight of 10 seeds, are first soaked for 48 h at 4 °C in deionized water as we noticed that this soaking improved the success of disinfection.
- Placement of germinated seeds in test tubes
- Open the bag containing sterile test tubes in a laminar flow cabinet. Discard the largest possible volume of glucose solution by pouring it into a sterile beaker. This step is very important to avoid contaminations.
- Then, add 15 ml of nutrient solution (N1 + P, see Recipes) in each tube of a rack with the 60 ml syringe. Cut the sealing film into strips of 25 x 150 mm (one per tube). Open the Petri dishes with germinated seeds by discarding the sealing film.
- Take a tube in one hand and move the filter paper away from the wall of the glass tube with a flame-sterilized spatula held in the other hand. Take a germinated seedling with tweezers and insert its root between the glass and the paper. Use the seeds with a root length of at least 7 cm and with teguments still covering the cotyledons. Gently press the filter paper against the root and close the tube with the sealing film by making several turns around the hypocotyl to completely close all the openings. Place the tube with the seedling (Figure 1) in another rack outside the laminar flow cabinet.
- Open the bag containing sterile test tubes in a laminar flow cabinet. Discard the largest possible volume of glucose solution by pouring it into a sterile beaker. This step is very important to avoid contaminations.
- Culture conditions of plants
- Place the tubes in a growth cabinet with controlled light and humidity. We generally use the following parameters to grow the plants: a 16/8 h light/dark cycle at 25 °C/20 °C, 60%/80% RH, CO2 concentration of about 350 x 10-6 dm-3 and a PAR (Photosynthetically Active Radiation) of approximately 400 μmol m-2 sec-1 (400-700 nm).
- Maintain the nutrient solution at 15 ml by adding regularly new nutrient solution under sterile conditions. Use a needle (18 G) equipped with a 40 mm-long piece of silicone tubing (int diam 1.14 mm). Several needles are prepared, placed in 125 ml polypropylene containers and autoclaved at 121 °C for 20 min.
- At the time of re-filling the test tubes, open the container under sterile conditions and connect a needle onto a sterile 60 ml Luer syringe filled with nutrient solution. Open first the PTFE microtube of a test tube by taking off the stopper (silicone tubing + burned 200 µl tip). Connect the open extremity of the microtube with the silicone tubing protruding from the syringe. Push the nutrient solution into the test tube until the wanted volume. Withdrawn the syringe and close back the PTFE microtube with its stopper.
- Place the tubes in a growth cabinet with controlled light and humidity. We generally use the following parameters to grow the plants: a 16/8 h light/dark cycle at 25 °C/20 °C, 60%/80% RH, CO2 concentration of about 350 x 10-6 dm-3 and a PAR (Photosynthetically Active Radiation) of approximately 400 μmol m-2 sec-1 (400-700 nm).
- Plant preparation for monitoring the fate of phosphate
- In our work (Torres-Aquino et al., 2017) we used 2-month old maritime pines. Six days before the incubation with the mycelia, the nutrient solution of each test tube is replaced by 30 ml of sterile CaSO4 (see Recipes). Six or seven test tubes are connected together by plugging one of the PTFE microtube in a home-made needle holder (Figure 3).
- The holder is connected to a sterile filter and the racks with the plants are transferred into the growth cabinet. The filter is then connected to an air pump to provide a sterile aeration to each test tube. After 5 days, the CaSO4 solution is replaced by 30 ml of interaction medium (see Recipes) for 24 h to acclimatize the roots to this new medium.
- In our work (Torres-Aquino et al., 2017) we used 2-month old maritime pines. Six days before the incubation with the mycelia, the nutrient solution of each test tube is replaced by 30 ml of sterile CaSO4 (see Recipes). Six or seven test tubes are connected together by plugging one of the PTFE microtube in a home-made needle holder (Figure 3).
- Incubation with the ectomycorrhizal fungus
- Disconnect the PTFE microtube from the connector and remove the sealing film. Take the plant out of the test tubes by hand. Add it to the 60 ml syringes containing a 32P labelled mycelium previously grown and rinsed as described in Becquer et al. (2017). In our work, we put three 2-month-old maritime pine seedlings in each syringe that contains 60 ml of interaction medium and a mycelium of the ectomycorrhizal basidiomycete Hebeloma cylindrosporum (about 0.2 g of fresh weight) suspended in the syringe by a nichrome wire (see Figure 4).
- Take a connector with PTFE microtubes (Figure 3) and insert one PTFE microtube into each syringe through the hole and connect the connector to an air pump. Adjust the flow rate of the pump and the position of the PTFE microtubes so as to obtain equivalent ventilation in all syringes. Add a glass funnel at the top of each syringe to minimize the evaporation of the medium. Incubate the plants and the fungi under laboratory light continuously for up to 48 h.
- To study the effect of plant on 32P efflux from the fungus, take 1 ml of interaction medium after 6, 24 and 48 h of incubation and measure the radioactivity as described in detail in our previous protocol (Becquer et al., 2017). Before sampling, do not forget to record the actual volume of interaction medium in the syringe. At the end of incubation, record the fresh weight of each mycelium and measure their radioactivity (for details, see Becquer et al., 2017). Take also each plant; blot it between filter paper sheets, separate roots from shoots and record their fresh weight. Then place the whole plant (roots + shoots) in a 20 ml scintillation vial and measure the radioactivity as described in Becquer et al. (2017).
- Disconnect the PTFE microtube from the connector and remove the sealing film. Take the plant out of the test tubes by hand. Add it to the 60 ml syringes containing a 32P labelled mycelium previously grown and rinsed as described in Becquer et al. (2017). In our work, we put three 2-month-old maritime pine seedlings in each syringe that contains 60 ml of interaction medium and a mycelium of the ectomycorrhizal basidiomycete Hebeloma cylindrosporum (about 0.2 g of fresh weight) suspended in the syringe by a nichrome wire (see Figure 4).
- Calculations with 32P labelled mycelia
- Refer to our previous protocol to correct the raw data (generally given in cpm = counts per minute) for decay (Becquer et al., 2017).
- Calculate the net amount of radioactivity released by the fungus after 6, 24 and 48 h (or any other incubation period) using the following equation written in Excel:
cpmt0-t6 = cpmt6 x [(60 + Vbs IMt6)/2]
cpmt24-t6 = cpmt24 x [(Vas IMt6 + Vbs IMt24/2]
cpmt24-t48 = cpmt48 x [(Vas IMt624 + Vbs IMt48)/2]
with:
cpmti= counts per minute measured in 1 ml of IM at time i
Vas IMti = volume (ml) of interaction medium after sampling at time i
Vbs IMti = volume (ml) of interaction medium before sampling at time i
Use these data to calculate the amounts of radioactivity lost by the fungus over the different incubation periods:
0-6 h = cpmt0-t6
0-24 h = cpmt0-t6 + cpmt6-t24
0-48 h = cpmt0-t6 + cpmt6-t24 + cpmt24-t48
- To calculate the whole amount of radioactivity lost by the fungus over 48 h, add the values of cpm measured in interaction medium (0-48 h) with those measured in plants.
- Add these previous values with those measured in the fungus before plant addition to get the total amount of radioactivity taken up by the fungus.
- Transform the values of cpm in Becquerel using the formula given in Becquer et al. (2017).
- Refer to our previous protocol to correct the raw data (generally given in cpm = counts per minute) for decay (Becquer et al., 2017).
Data analysis
When the fungus is labelled with 32P (see Becquer et al., 2017), the number of replicates per treatment should be at least 6 and the experiments should be repeated twice. Results can be expressed either as the percentage of initial radioactivity lost by the fungus or per g of fungal fresh weight. The normality of data is tested using the Kolmogorov Smirnov test and, where necessary, the data are either square root or log10 transformed prior to analysis to meet the assumptions of ANOVA. The effect of incubation time (e.g., 6, 24 or 48 h) and of the biological conditions (the fungus alone versus the fungus with the plant) can be assessed using one way- or two way-ANOVA.
Notes
- In the disinfection step of maritime pine seeds, the person must wear gloves to avoid any burning from skin contact with pure, concentrated H2O2.
- The seeds must have a good contact with H2O2 for efficient disinfection.
- At the time of seedling transfer into the test tube, it is very important that they have their tegument covering the cotyledons. Without this tegument, the young seedling will dry very quickly and die.
- In case the tegument has been lost, the seedlings can be protected from air drying by covering with a filter paper, well-moistened with deionized water.
- Depending on the number of plants required for the experiments, it is possible to place 2 germinated seeds side by side in the tube. In this case, it is necessary to monitor the level of the nutrient solution very frequently.
- Since the risk of contamination of filter paper with bacteria or saprophytic fungi is never zero, we usually prepare 10% more plants than needed.
Recipes
- Agar plates for germination
- Prepare the glucose solution by dissolving 2 g of D-glucose in 1 L of deionized water
- Take two 1 L glass bottles, add 7.5 g of Agar-agar and pour 500 ml of glucose solution in each bottle
- Sterilize the agar medium at 121 °C for 20 min
- Pour the cooled medium (55-60 °C) in Petri dishes 90 mm diameter
- Prepare the glucose solution by dissolving 2 g of D-glucose in 1 L of deionized water
- 0.1 M Ca(OH)2 solution
- Pour 100 ml of deionized water in a 250 ml-glass bottle
- Add 0.74 g of Ca(OH)2 and shake
- The powder may not completely dissolve and the solution must be shaken by hand to resuspend the powder before use
- Store at room temperature
- Pour 100 ml of deionized water in a 250 ml-glass bottle
- Trace elements (1,000 ml)
3.08 g MnSO4·H2O
4.41 g ZnSO4·7H2O
2.82 g H3BO3
0.98 g CuSO4·5H2O
0.29 g Na2MoO4·2H2O
Add deionized H2O to 1,000 ml, store at 4 °C
- Mineral salt base solutions (100 ml)
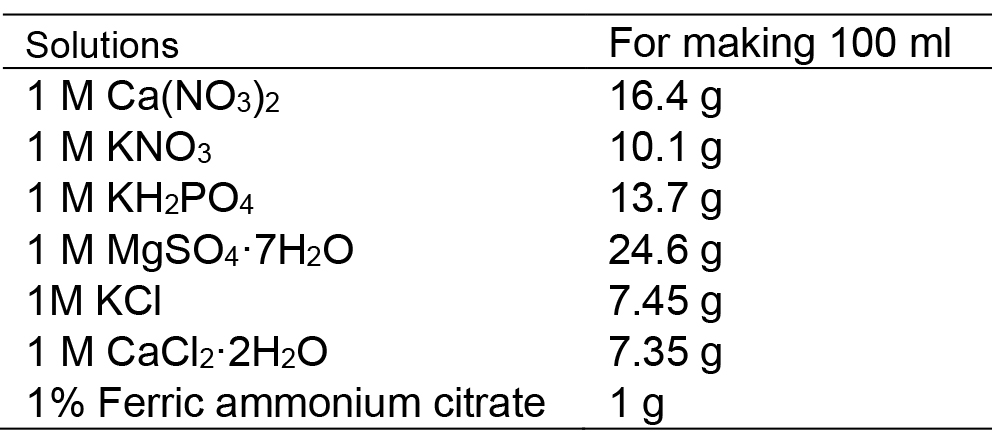
All these solutions are stored at 4 °C
- N1 + P solution (1,000 ml)
- Add in a 1 L-beaker approximately 500 ml of deionized water and the following volumes of mineral base solutions:
0.2 ml Ca(NO3)2
0.6 ml KNO3
0.2 ml KCl
0.2 ml KH2PO4
0.5 ml ferric ammonium citrate
- Add also trace elements 0.2 ml
- Complete the volume to 1 L and adjust the pH to 5.5
- Sterilize the solution at 121 °C for 20 min
- Add in a 1 L-beaker approximately 500 ml of deionized water and the following volumes of mineral base solutions:
- CaSO4 solution (0.2 mM)
- Add in a 1 L-beaker 1,000 ml of deionized water and 34.4 mg of calcium sulphate
- Shake the solution carefully and adjust the pH to 5.5 if necessary with 0.1 N H2SO4 or 0.1 M Ca(OH)2
- Sterilize the solution at 121 °C for 20 min
- Add in a 1 L-beaker 1,000 ml of deionized water and 34.4 mg of calcium sulphate
- Interaction medium (3,000 ml)
- Add in a 3 L-beaker approximately 1.5 L of deionized water and the following volumes of mineral base solutions:
0.6 ml MgSO4
1.5 ml CaCl2
3.2 g of MES (final concentration of 5 mM)
1.82 g of TRIS (final concentration of 5 mM)
- Complete to 3 L with deionized water and adjust the pH to 5.5 with 1 N H2SO4
- Pour 1.5 L of medium into 2 L glass bottles and sterilize by autoclaving (20 min, 121 °C)
- Add in a 3 L-beaker approximately 1.5 L of deionized water and the following volumes of mineral base solutions:
Acknowledgments
This research was supported by INRA (France) through annual funding devoted to their researchers and a fellowship through a Contract for Young Scientist (CJS) granted to Adeline Becquer, and by CONACYT (Mexico) through a Ph-D fellowship granted to Margarita Torres-Aquino. The protocol is adapted from our previous work (Plassard et al., 1994; Torres-Aquino et al., 2017). We also thank the three anonymous reviewers for their helpful comments to improve the protocol.
References
- Becquer, A., Torres-Aquino, M., Le Guernevé, C., Amenc, L.K., Trives-segura, C., Staunton, S., Quiquampoix, H. and Plassard, C. (2017). A Method for Radioactive labelling of Hebeloma cylindrosporum to study plant-fungus interactions. Bio Protoc 7(20): e2576.
- Cairney, J. W. G. (2011). Ectomycorrhizal fungi: the symbiotic route to the root for phosphorus in forest soils. Plant Soil 344: 51-71.
- Plassard, C., Barry, D., Eltrop, L. and Mousain, D. (1994). Nitrate uptake in maritime pine (Pinus pinaster) and the ectomycorrhizal fungus Hebeloma cylindrosporum: effect of ectomycorrhizal symbiosis. Can J Bot 72: 189-197.
- Plassard, C. and Dell, B. (2010). Phosphorus nutrition of mycorrhizal trees. Tree Physiol 30(9): 1129-1139.
- Smith, S. E. and Read, D. J. (2008). Mycorrhizal symbiosis. 3rd edition. Academic Press.
- Smith, S. E., Anderson, I. C. and Smith, F. A. (2015). Mycorrhizal associations and phosphorus acquisition: from cells to ecosystems. Annual Plant Reviews 48: 409-440.
- Torres-Aquino, M., Becquer, A., Le Guerneve, C., Louche, J., Amenc, L. K., Staunton, S., Quiquampoix, H. and Plassard, C. (2017). The host plant Pinus pinaster exerts specific effects on phosphate efflux and polyphosphate metabolism of the ectomycorrhizal fungus Hebeloma cylindrosporum: a radiotracer, cytological staining and 31P NMR spectroscopy study. Plant Cell Environ 40(2): 190-202.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Becquer, A., Torres-Aquino, M., Le Guernevé, C., Amenc, L. K., Trives-Segura, C., Staunton, S., Quiquampoix, H. and Plassard, C. (2017). Establishing a Symbiotic Interface between Cultured Ectomycorrhizal Fungi and Plants to Follow Fungal Phosphate Metabolism. Bio-protocol 7(20): e2577. DOI: 10.21769/BioProtoc.2577.
Category
Plant Science > Plant physiology > Nutrition
Cell Biology > Tissue analysis > Physiology
Microbiology > Microbe-host interactions > Fungus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










