- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Tracking Endocytosis and Intracellular Trafficking of Epitope-tagged Syntaxin 3 by Antibody Feeding in Live, Polarized MDCK Cells
Published: Vol 8, Iss 3, Feb 5, 2018 DOI: 10.21769/BioProtoc.2453 Views: 9513
Reviewed by: Gal HaimovichAlexandros KokotosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Studying Cellular Focal Adhesion Parameters with Imaging and MATLAB Analysis
Ling-Yea Yu [...] Feng-Chiao Tsai
Nov 5, 2023 1879 Views

Automated Layer Analysis (ALAn): An Image Analysis Tool for the Unbiased Characterization of Mammalian Epithelial Architecture in Culture
Christian Cammarota [...] Tara M. Finegan
Apr 20, 2024 4222 Views

Accurate Identification of Cell Cycle Stages in RPE1 Cells Using the ImmunoCellCycle-ID Method
Syon Reddy [...] Aussie Suzuki
Aug 5, 2025 1870 Views
Abstract
The uptake and trafficking of cell surface receptors can be monitored by a technique called ‘antibody-feeding’ which uses an externally applied antibody to label the receptor on the surface of cultured, live cells. Here, we adapt the traditional antibody-feeding experiment to polarized epithelial cells (Madin-Darby Canine Kidney) grown on permeable Transwell supports. By adding two tandem extracellular Myc epitope tags to the C-terminus of the SNARE protein syntaxin 3 (Stx3), we provided a site where an antibody could bind, allowing us to perform antibody-feeding experiments on cells with distinct apical and basolateral membranes. With this procedure, we observed the endocytosis and intracellular trafficking of Stx3. Specifically, we assessed the internalization rate of Stx3 from the basolateral membrane and observed the ensuing endocytic route in both time and space using immunofluorescence microscopy on cells fixed at different time points. For cell lines that form a polarized monolayer containing distinct apical and basolateral membranes when cultured on permeable supports, e.g., MDCK or Caco-2, this protocol can measure the rate of endocytosis and follow the subsequent trafficking of a target protein from either limiting membrane.
Keywords: Antibody-feeding assayBackground
The SNARE protein Syntaxin 3 (Stx3) is known to establish apical-basolateral polarity in polarized epithelial cells (Low et al., 1996; Delgrossi et al., 1997; Weimbs et al., 1997; Low et al., 1998; Li et al., 2002; Low et al., 2006). Apical localization of Stx3 depends on a conserved targeting motif near the N-terminus of the protein (ter Beest et al., 2005; Sharma et al., 2006). A fraction of Stx3 is also known to localize to late endosomes/lysosomes in Madin-Darby Canine Kidney (MDCK) cells, which form tight-junctions, establish apical and basolateral polarity, and adopt a columnar morphology when grown to confluence. To determine the origin of this population of Stx3 and to investigate its endosomal trafficking in polarized MDCK cells, we designed an ‘antibody-feeding assay’ protocol. Using this assay and other experiments, we have shown that Stx3 is ubiquitinated at lysine residues in a basic juxtamembrane region and that ubiquitination facilitates the endocytosis of Stx3 from the basolateral membrane leading to trafficking to intraluminal vesicles of multivesicular bodies, and eventually secretion with exosomes (Giovannone et al., 2017). A non-ubiquitinatable mutant (Stx3-5R) exhibits decreased endosomal trafficking and exosomal secretion (Giovannone et al., 2017). Using an antibody-feeding assay, we can monitor where Stx3 is trafficked after being delivered to the apical or basolateral membrane. A cassette containing two Myc epitope tags and one hexa-histidine tag was added to the C-terminus of Stx3 by molecular cloning. These tags are exposed to the extracytoplasmic side of the plasma membrane when tagged Stx3 is present on the surface in transfected cells. We have previously shown that these C-terminal Myc2-His6 tags are accessible to the 9E10 anti-c-Myc monoclonal antibody and do not interfere with the known surface polarity of several syntaxins (Low et al., 2000; Kreitzer et al., 2003; Low et al., 2006; Sharma et al., 2006; Reales et al., 2011; Giovannone et al., 2017). Epitope-tagged Stx3 is stably expressed in MDCK cells using a tetracycline-controlled transcriptional activation system which allows using uninduced cells as a negative control. Cells are cultured on Transwell permeable membranes until fully polarized (Figure 1), incubated with anti-c-Myc antibody, and harvested for analysis by immunofluorescence microscopy at various time points. Cells were also incubated with anti-M6PR antibody (late endosomal marker). Lastly, cells were stained with DAPI and secondary antibodies against the 9E10 anti-c-Myc monoclonal antibody (for Stx3) and anti-M6PR antibody. 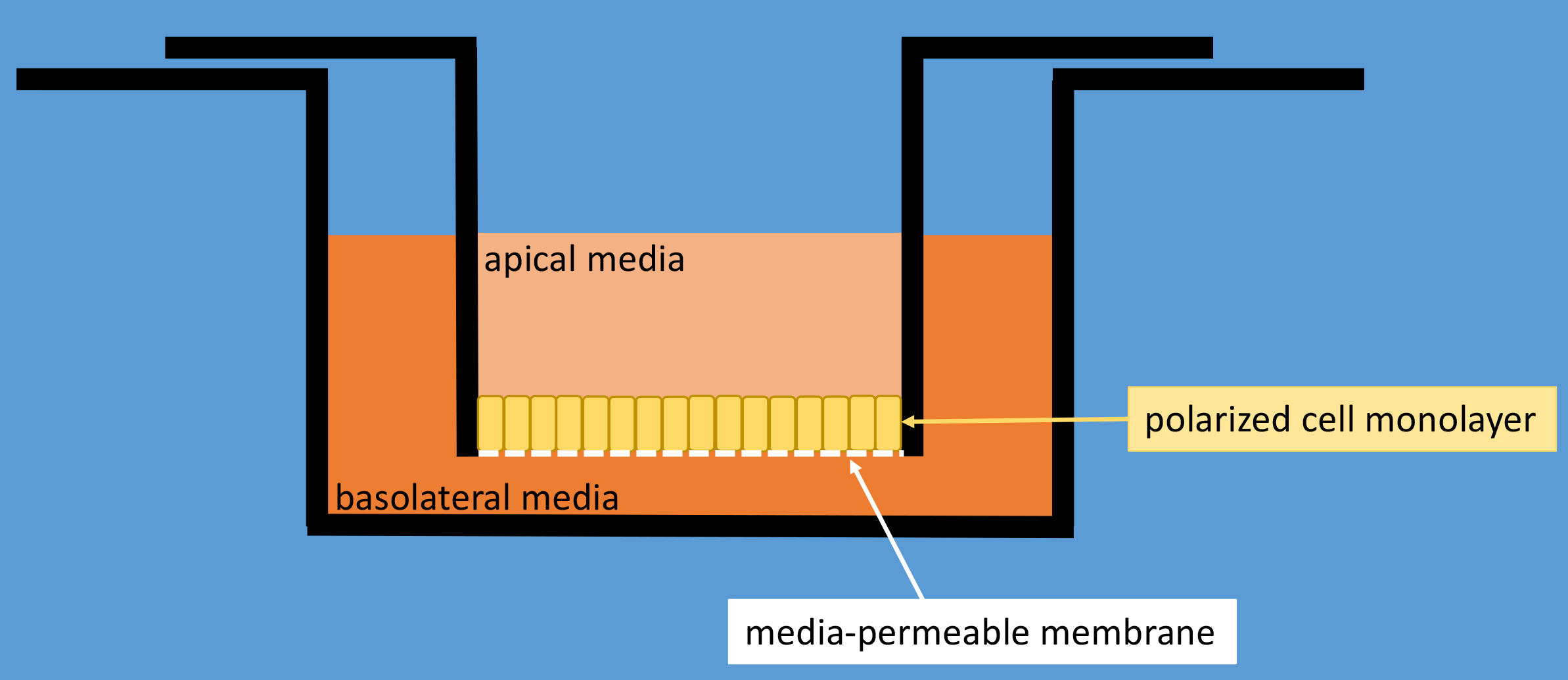
Figure 1. Schematic drawing of a Transwell polycarbonate membrane cell culture insert inside a well of a typical 12-well cell culture dish. Cells are cultured on top of the membrane and will form a tight monolayer that seals off the polycarbonate membrane thereby separating the apical media compartment from the basolateral media compartment.
Materials and Reagents
- 10 cm cell culture dishes (Corning, Falcon®, catalog number: 353003 )
- 10 ml serological pipette (VWR, catalog number: 89130-898 )
- 50 ml conical tubes (Corning, catalog number: 430290 )
- 12-well cell culture plates with 0.4 µm pore polycarbonate Transwell supports (Corning, catalog number: 3401 )
- 12-well cell culture plates (Corning, Costar®, catalog number: 3512 )
- Razor blades (VWR, catalog number: 55411-050 )
- Paper towels
- Kimwipes (KCWW, Kimberly-Clark, catalog number: 34120 )
- Parafilm (BEMIS, catalog number: PM996 )
- Plastic pencil box with lid (VWR, catalog number: 500003-109 )
Manufacturer: Janitorial Supplies, catalog number: AVT34104 . - Microscope slides (any standard slides from any supplier will be fine)
- Micro cover glasses, 18 x 18 mm, No. 1 thickness is 0.13 to 0.17 mm (VWR, catalog number: 48366-045 )
- Madin-Darby Canine Kidney (MDCK) cells stably expressing C-terminally Myc2-His6-tagged Stx3
Note: Madin-Darby Canine Kidney (MDCK) cells stably expressing C-terminally Myc2-His6-tagged Stx3 using a doxycycline-inducible expression system have been described in detail in Sharma et al., 2006. The parental cell line, expressing the TET transactivator is required to produce doxycycline-inducible, stably transfected cells for one’s gene of interest and have been generated in the laboratory of the senior author (TW). Cells may be requested from the authors. Original MDCK cells and several subclones are also available from ATCC (ATCC, catalog number: CCL-34 and others) but these cells may differ in some characteristics from those used here. - Sterile 1x Dulbecco’s phosphate buffered saline (DPBS) without calcium or magnesium (Mediatech, catalog number: 21-031-CV )
- Sterile 0.25% trypsin/EDTA (Mediatech, catalog number: 22-053-CI )
- 4% buffered formalin solution (Sigma-Aldrich, catalog number: HT5012 )
- Prolong Gold anti-fade reagent plus DAPI (Thermo Fisher Scientific, InvitrogenTM, catalog number: P36934 )
- Sterile Minimum Essential Medium without glutamine (Mediatech, catalog number: 15-010-CV )
- Sterile L-glutamine 100x (Mediatech, catalog number: 25-005-CI )
- Penicillin/Streptomycin 100x (Mediatech, catalog number: 30-002-CI )
- Fetal bovine serum (FBS) (Omega Scientific, catalog number: FB-11 )
- Doxycycline monohydrate (tetracycline analog) (Sigma-Aldrich, catalog number: D1822 )
- HEPES (Fisher Scientific, catalog number: BP310 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2153 )
- Anti-c-Myc monoclonal antibody, clone 9E10
Note: Hybridoma cells from The Developmental Studies Hybridoma Bank (http://dshb.biology.uiowa.edu). Prepare mouse ascites using a commercial vendor. - Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: A5666 )
Note: This product has been discontinued. - L-glycine (Fisher Scientific, catalog number: BP381-5 )
- Normal Donkey serum (LAMPIRE Biological Labs, catalog number: 7332100 )
- Triton X-100 (Fisher Scientific, catalog number: BP151-500 )
- Mannose-6-Phosphate Receptor antibody (kind gift from William Brown, Cornell University)
- Fish skin gelatin (Sigma-Aldrich, catalog number: G7765 )
- Donkey anti-mouse DyLight 488 (Jackson ImmunoResearch Laboratories, catalog number: 715-485-150 )
Note: This product has been discontinued. - Donkey anti-rabbit DyLight 594 (Jackson ImmunoResearch Laboratories, catalog number: 711-515-152 )
Note: This product has been discontinued. - Complete media (see Recipes)
- Serum-free media (see Recipes)
- 1 mg/ml doxycycline stock solution (see Recipes)
- MEM ETC (see Recipes)
- MEM ETC containing anti-c-Myc antibody (see Recipes)
- Quench solution (see Recipes)
- Block and Permeabilization buffer (see Recipes)
- Primary antibody solution (see Recipes)
- Washing solution (see Recipes)
- Secondary antibody solution (see Recipes)
Equipment
- BSL-2 hood (Nuaire, class II)
- Tissue culture incubator at 37 °C and 5% CO2 (Thermo Fisher Scientific, Thermo ScientificTM, model: HeracellTM 150 )
- Orbital shaker (Bellco)
- Hemocytometer (Hausser Scientific, catalog number: 3200 )
- Surgical scissors 140 mm (Dunrite Instruments, catalog number: 140940 )
- Dissecting forceps, fine tip, non-serrated
- Olympus Fluoview FV1000S (Olympus, model: Fluoview FV1000 ) Spectral Laser Scanning Confocal microscope using an Olympus UPLFLN 60x oil-immersion objective
- Cold room (4 °C)
Software
- Adobe Photoshop (Adobe Systems Inc.)
Procedure
Notes:
- Perform Procedures A and B using aseptic technique in a BSL-2 biosafety cabinet.
- For Transwells, the upper chamber volume is 0.5 ml; the lower chamber volume is 1 ml.
- The volume of any solution added to membrane cut-out is 50 µl.
- Seed cells
- Starting with a 10 cm dish of MDCK cells in culture, aspirate complete media (see Recipes) and add 10 ml of DPBS without calcium or magnesium.
- Aspirate DPBS and add 1 ml of 0.25% trypsin/EDTA, place in a 37 °C incubator with 5% CO2 for 5 min occasionally tapping dish to dislodge cells.
- Add 9 ml of complete medium to dislodged cells in plate. Using a 10 ml serological pipette, pipette the cell suspension multiple times to detach the cells from each other.
- Use a hemocytometer to count the single-cell cell suspension.
- Dilute the cell suspension to a concentration of 50,000 cells/ml in complete media.
- Add 0.5 ml of cell dilution to the upper chamber of Transwell.
- Add 1 ml of complete media to the lower chamber of Transwell.
- Next day, change media to serum-free media (see Recipes) in the upper chamber. Keep complete media (serum-replete) in the lower chamber.
- After four days, add serum-free media containing doxycycline to the upper chamber and add complete media containing doxycycline to the lower chamber (dilute doxycycline stock solution [see Recipes] 1:20,000 to 50 ng/ml); incubate overnight.
- Starting with a 10 cm dish of MDCK cells in culture, aspirate complete media (see Recipes) and add 10 ml of DPBS without calcium or magnesium.
- Antibody feeding
- Replace media on Transwells with ice-cold MEM ETC (see Recipes) and keep on ice.
- Add ice-cold MEM ETC containing anti-c-Myc antibody (see Recipes) to wells of a new 12-well plate and place on ice.
- Transfer each Transwell insert containing your cells to the new 12-well plate containing antibody on ice.
- Incubate on ice in the cold room for 20 min with gentle shaking.
- Remove plate from the cold room and aspirate antibody media from the lower chamber.
- Wash lower chambers with ice-cold MEM ETC three times for 5 min on ice in cold room with gentle shaking.
- Transfer each Transwell insert to a new 12-well plate containing pre-warmed MEM ETC in each well. Replace apical media with pre-warmed MEM ETC. Move to a 37 °C incubator.
- Incubate wells for the desired time, remove from the incubator, place in a new 12-well plate containing ice-cold MEM ETC, and store on ice until all time points are completed.
- Wash one time with ice-cold MEM ETC for 5 min on ice in cold room with gentle shaking. Remove media.
- Add 4% buffered formalin solution to upper and lower chambers; incubate at room temperature for 15 min with gentle shaking.
- Aspirate formalin and add quench solution (see Recipes) to both chambers and incubate at room temperature for 10 min with gentle shaking.
- Aspirate quench solution and wash both chambers with PBS three times.
- Add block and permeabilization buffer (see Recipes) to both chambers and incubate at 37 °C for 30 min.
- Replace media on Transwells with ice-cold MEM ETC (see Recipes) and keep on ice.
- Primary antibody (M6PR antibody) incubation
- Aspirate blocking buffer, remove Transwell inserts from the plate, and place upside-down (apical side facing downward) on bench top.
- Use a new razor blade to cut three sides of a rectangle in the Transwell membrane.
- Gently grab the square membrane with forceps and cut the rectangle from the Transwell support using surgical scissors. Clip a small piece from the upper right corner to be able to keep track of which side the cells are on. (Video 1)
Video 1. Demonstration of the procedure to cut out the Transwell membrane using a razor blade. The lid of the multiwall dish is covered with Parafilm and used as a support surface. - Place the membrane cut-out, cell side up, onto a sheet of Parafilm placed in a lid of a 12-well plate with each well labeled.
- Gently add 50 µl of primary antibody solution (see Recipes) on top of membrane cut-out.
- Place samples in a plastic pencil box lined with damp paper towels to create a humid chamber and incubate overnight in the cold room.
- Aspirate blocking buffer, remove Transwell inserts from the plate, and place upside-down (apical side facing downward) on bench top.
- Secondary antibody incubation
- Aspirate primary antibody solution, transfer membranes to a new 12-well plate, and wash four times each for 5 min with washing solution (see Recipes).
- Add secondary antibody solution (see Recipes) to membranes, incubate at 37 °C in the humid chamber for 1 h.
- Aspirate secondary antibody solution and wash three times 5 min with washing solution.
- Wash once with PBS.
- Aspirate PBS, and post-fix by adding 4% buffered formalin solution, and incubate at room temperature for 10 min.
- Aspirate formalin, wash one time with PBS quickly, and another time for 5 min.
- Aspirate PBS, use forceps to dip membrane in ddH2O for 1 sec. Remove from ddH2O, wick water away by gently touching membrane to a Kimwipe, and place on microscope slide cell-side up. Keep membrane flat the entire time preventing it from folding back on itself.
- Add a drop of mounting medium (Prolong Gold anti-fade reagent plus DAPI) to each membrane.
- Gently add rectangular coverslip to membranes avoiding air bubbles.
- Store slides in the dark at room temperature for 48 h to harden mounting media.
- Aspirate primary antibody solution, transfer membranes to a new 12-well plate, and wash four times each for 5 min with washing solution (see Recipes).
Data analysis
- Analyze using a confocal microscope.
- Acquire high-quality images of representative fields from focal planes corresponding to different areas of the cell mono-layer (nuclear level, apical cytoplasm, apical plasma membrane).
- Acquire corresponding images from each time point.
- Assemble micrographs in Adobe Photoshop.
- Results in Figure 2.
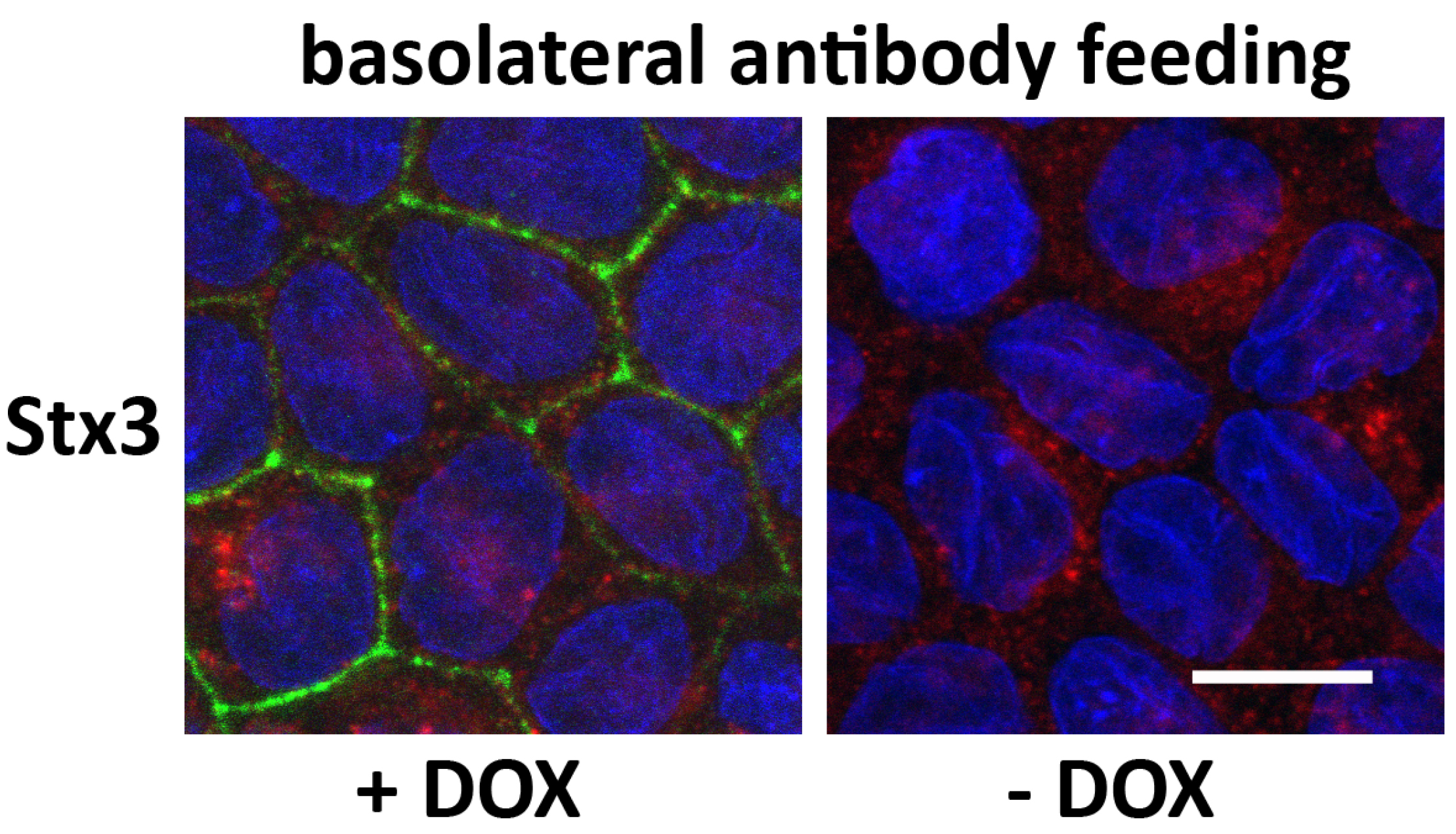
Figure 2. Example of immunofluorescence microscopy of a basolateral antibody feeding experiment. MDCK cells stably transfected for wild-type Stx3 were grown at to post-confluence for 4 days on Transwell filters, and then induced with DOX for 16 h. Anti-myc antibody (9E10 ascites) was added to the basolateral media and incubated at 4 °C before being washed off and incubated at 37 °C for 5 min. Cells were fixed and stained for Stx3 (myc), green; M6PR, red; and nuclei (DAPI), blue. Scale bar = 10 μm.
Notes
To perform the apical antibody-feeding experiment, repeat the protocol but add MEM ETC containing anti-c-Myc ascites to the upper chamber instead of the lower chamber.
Recipes
- Complete media (500 ml)
To a new bottle of Minimum Essential Media, add:
5 ml L-glutamine
5 ml penicillin/streptomycin
50 ml of FBS - Serum-free media (500 ml)
To a new bottle of Minimum Essential Media, add:
5 ml L-glutamine
5 ml penicillin/streptomycin - 1 mg/ml doxycycline stock solution (store at -20 °C)
Add 50 mg of doxycycline to 50 ml of ddH2O - MEM ETC (Minimum Essential Medium + 20 mM HEPES + 0.6% BSA + Pen/Strep)
- To Minimum Essential Medium with penicillin/streptomycin add 1 M HEPES-NaOH (pH 7.4) to a final concentration of 20 mM
- Add BSA to a final concentration of 0.6%
- To Minimum Essential Medium with penicillin/streptomycin add 1 M HEPES-NaOH (pH 7.4) to a final concentration of 20 mM
- MEM ETC containing anti-c-Myc antibody ascites (1:200)
Add 50 µl of mouse ascites with 9E10 anti-c-Myc antibody to 10 ml of MEM ETC - Quench solution (75 mM NH4Cl + 20 mM L-glycine)
200.6 mg of NH4Cl
75.1 mg L-glycine
Bring to volume 50 ml with PBS - Block and Permeabilization buffer (PBS containing 0.2% Triton X-100, 5% Normal Donkey serum)
1 ml of Normal Donkey serum
2 ml of 10x PBS
0.4 ml of 10% Triton X-100 stock
Bring to volume 20 ml with ddH2O - Primary antibody solution
Add 3 µl of anti-M6PR (1:200) to 600 µl of Block and Permeabilization buffer - Washing solution (PBS containing 0.05% Triton X-100, 0.7% fish skin gelatin)
70 mg of fish skin gelatin
50 µl of 10% Triton X-100
1 ml of 10x PBS
Bring to volume 10 ml with ddH2O - Secondary antibody solution
Add 1 µl of donkey anti-mouse DyLight 488 (1:1,000) and 1 µl of donkey anti-rabbit DyLight 594 (1:1,000) to 1 ml of Block and Permeabilization buffer
Acknowledgments
This work was supported by grants from the NIH (DK62338), and the California Cancer Research Coordinating Committee to T.W., by a grant BFU2012-35067 from the Spanish Ministry of Economy and Competitiveness (MINECO) to A.F.-R., and a Postdoctoral Fellowship from the Spanish Ministry of Education and Science to E.R. The authors declare no conflicts of interest or competing interests.
References
- Delgrossi, M. H., Breuza, L., Mirre, C., Chavrier, P. and Le Bivic, A. (1997). Human syntaxin 3 is localized apically in human intestinal cells. J Cell Sci 110 (Pt 18): 2207-2214.
- Giovannone, A. J., Reales, E., Bhattaram, P., Fraile-Ramos, A. and Weimbs, T. (2017). Monoubiquitination of syntaxin 3 leads to retrieval from the basolateral plasma membrane and facilitates cargo recruitment to exosomes. Mol Biol Cell 28(21): 2843-2853.
- Kreitzer, G., Schmoranzer, J., Low, S. H., Li, X., Gan, Y., Weimbs, T., Simon, S. M. and Rodriguez-Boulan, E. (2003). Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat Cell Biol 5(2): 126-136.
- Li, X., Low, S. H., Miura, M. and Weimbs, T. (2002). SNARE expression and localization in renal epithelial cells suggest mechanism for variability of trafficking phenotypes. Am J Physiol Renal Physiol 283(5): F1111-1122.
- Low, S. H., Chapin, S. J., Weimbs, T., Komuves, L. G., Bennett, M. K. and Mostov, K. E. (1996). Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell 7(12): 2007-2018.
- Low, S. H., Chapin, S. J., Wimmer, C., Whiteheart, S. W., Komuves, L. G., Mostov, K. E. and Weimbs, T. (1998). The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Cell Biol 141(7): 1503-1513.
- Low, S. H., Miura, M., Roche, P. A., Valdez, A. C., Mostov, K. E. and Weimbs, T. (2000). Intracellular redirection of plasma membrane trafficking after loss of epithelial cell polarity. Mol Biol Cell 11(9): 3045-3060.
- Low, S. H., Vasanji, A., Nanduri, J., He, M., Sharma, N., Koo, M., Drazba, J. and Weimbs, T. (2006). Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol Biol Cell 17(2): 977-989.
- Reales, E., Sharma, N., Low, S. H., Folsch, H. and Weimbs, T. (2011). Basolateral sorting of syntaxin 4 is dependent on its N-terminal domain and the AP1B clathrin adaptor, and required for the epithelial cell polarity. PLoS One 6(6): e21181.
- Sharma, N., Low, S. H., Misra, S., Pallavi, B. and Weimbs, T. (2006). Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J Cell Biol 173(6): 937-948.
- ter Beest, M. B., Chapin, S. J., Avrahami, D. and Mostov, K. E. (2005). The role of syntaxins in the specificity of vesicle targeting in polarized epithelial cells. Mol Biol Cell 16(12): 5784-5792.
- Weimbs, T., Low, S. H., Chapin, S. J. and Mostov, K. E. (1997). Apical targeting in polarized epithelial cells: There's more afloat than rafts. Trends Cell Biol 7(10): 393-399.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Giovannone, A. J., Reales, E., Bhattaram, P., Fraile-Ramos, A. and Weimbs, T. (2018). Tracking Endocytosis and Intracellular Trafficking of Epitope-tagged Syntaxin 3 by Antibody Feeding in Live, Polarized MDCK Cells. Bio-protocol 8(3): e2453. DOI: 10.21769/BioProtoc.2453.
Category
Cell Biology > Cell imaging > Fixed-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










