- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Wheat Coleoptile Inoculation by Fusarium graminearum for Large-scale Phenotypic Analysis
Published: Vol 7, Iss 15, Aug 5, 2017 DOI: 10.21769/BioProtoc.2439 Views: 11750
Reviewed by: Gazala AmeenVinay PanwarAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In Vitro Hyphal Branching Assay Using Rhizophagus irregularis
Takaya Tominaga and Hironori Kaminaka
Aug 20, 2024 2315 Views

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2634 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1568 Views
Abstract
The ascomycete fungus Fusarium graminearum is a destructive fungal pathogen of wheat, barley and maize. Although reverse genetics and homologous recombination gene deletion methods have generated thousands of gene deletion mutants of F. graminearum, evaluating virulence of these fungal mutants is still a rate-limiting step. Here we present a protocol for inoculation of wheat coleoptiles with conidial suspensions for large-scale phenotypic analysis, and describe how it can also be used to assess fungal infectious growth and symptom developmentat a cellular scale. The inoculation method described in this protocol provides highly reproducible results in wheat coleoptile infection by F. graminearum.
Keywords: Fusarium graminearumBackground
Fusarium graminearum (previously also called Gibberella zeae) is a destructive pathogen that upon infection is responsible for causing Fusarium head blight (FHB) and seedling blight on cereal crops, as well as stalk and ear rot on maize (Dal Bello et al., 2002; Bai and Shaner, 2004; Kazan et al., 2012). Extensive molecular and genetic studies have been performed to investigate the interaction between F. graminearum and wheat. Given the availability of an efficient genetic transformation system and the well annotated genome, hundreds of F. graminearum genes have been investigated for their roles in vegetative growth, sexual development, secondary metabolism, stress responses and even virulence on host (Jia and Tang, 2015). However, only a few fungal effectors (e.g., FGL1 and deoxynivalenol) and host resistance genes (e.g., wheat Fhb1) have been identified (Proctor et al., 1995; Blümke et al., 2014; Rawat et al., 2016).
F. graminearum has been reported to lack pathogen-specialized patterns that typically induce gene-for-gene-mediated resistance in the host (van Eeuwijk et al., 1995). Furthermore, gene redundancy and functional complementation make assigning definitive virulence roles to pathogen genes achallenge. In addition, the traditional wheat head infection assay is limited due to seasonal, temporal and spatial factors. The distinct structures (rachis, paleas, lemmas, caryopses and glumes) and diverse features of wheat florets also make it difficult to track the infection progress of F. graminearum.
Previously, we used a modified wheat coleoptile infection assay and microscopic inspection to study F. graminearum infection inside host tissue (Zhang et al., 2012). Unlike the wheat head infection assay, there are few temporal and spatial constraints for the seedling infection system. The wheat coleoptile infection assay is performed in a growth chamber that can hold up to two hundred 24-well plates, which means more than 100 genes can be evaluated for their roles in virulence (three independent transgenic lines for each tested gene, and at least twelve seedlings for inoculation of each fungal strain). The time required to complete the assay is short: ten days for seed germination (Figure 1), inoculation (Figure 2) and examination of lesion size (Figure 3). The structure of wheat coleoptile is simple: the annular coleoptile comprises similar cells and two vascular bundles (Figure 2D) and is easy to inspect microscopically (Zhang et al., 2012). Seven genes were identified required for full virulence of F. graminearum on wheat coleoptile, and several of which were also required for wheat head infection (Zhang et al., 2012) and even maize stalk infection (Zhang et al., 2016).
Materials and Reagents
- Pipette tips (Corning, Axygen®, catalog number: T-300-R-S )
- Disposable paper towels (Vinda Classic Blue 1800, Vinda, catalog number: V4028 )
- 24-well cell culture plate (Corning, Costar®, catalog number: 3524 )
- Sterile toothpick
- Microtubes (Corning, Axygen®, catalog number: MCT-150-C )
- Medical-grade gauze (regular cotton yarn, Shanghai Honglong Medical Material Company)
- Medical-grade absorbent cotton (Shanghai Honglong Medical Material Company)
- Enamel tray (20x 30 x 5 cm)
- Glass slides (specifications: 76.2 x 25.4 mm, thickness: 1.0-1.2 mm) (Livingstone, catalog number: 7105-1 )
- Cover slips (specifications: 24 x 50 mm, thickness: 0.13-0.16 mm) (CITOTEST LABWARE MANUFACTURING, catalog number: 10212450C )
- Fungal strains: F. graminearum wild-type strain PH-1 (NRRL 31084), AmCyanPH-1 (Zhang et al., 2012) and gene deletion mutants of PH-1 (Zhang et al., 2012 and 2016)
- Plant material: Wheat (Triticum aestivum) cultivar Zhongyuan 98-68 (susceptible to F. graminearum and widely cultured in Henan, China)
- Sterile water
- V8 vegetable juice (CAMPBELL, catalog number: V8® ORIGINAL )
- Calcium carbonate (CaCO3)
- Agar powder
- Mung beans
- V8 juice agar medium (He et al., 2016; see Recipes)
- Mung bean liquid medium (He et al., 2016; see Recipes)
Equipment
- 500 ml flask
- Pipettes (Eppendorf)
- Growth chamber (Ningbo Jiangnan Instrument Factory, model: RXZ-1000 )
- Mould cultivation cabinet (Yiheng, model: MJ-150-I )
- Biological safety cabinet (ESCO Micro, model: FHC-1200A )
- Constant temperature shaker (Taicang, model: DHZ-DA )
- Hemocytometer (0.10 mm, 1/400 mm2) (QIUJING, model: XB-K-25 )
- Fluorescent microscope (Olympus, model: Olympus BX51 )
- Confocal microscope (Olympus, model: Fv10i )
- Centrifuge (Beckman Coulter, model: Avanti J-E Series )
- Camera (Canon, model: EOS 7D )
- Autoclave
- Rule
Software
- ImageJ (http://rsbweb.nih.gov/ij/index.html)
- Microsoft Excel
Procedure
- Preparation of wheat seedlings
- Soak the wheat seeds at room temperature in a 500 ml flask, and rinse with running water overnight (Figure 1A).
Note: Prepare about 20 seeds for each inoculation treatment to make sure at least 12 germinated seedlings can be used for each inoculation treatment in one experiment. For example, to examine the virulence of two strains of mutant F. graminearum, along with one wild-type strain and mock inoculation, 80 seeds need to be soaked. - Place the imbibed seeds in a medical enamel tray containing 2 layers of wet gauze (Figure 1B) and germinate in the dark for 1 day at 25 °C in a growth chamber.
Note: Cover the tray with the lid to keep the gauze wet during germination. - Put a small piece of paper towel in each well of 24-well cell culture plates and drench with water. Transplant the germinated seeds to 24-well cell culture plates, one seed per well (Figure 1C).
- Grow the plants in the growth chamber for 1 day under controlled environment conditions at 25 °C with a 12 h light/12 h dark photoperiod.
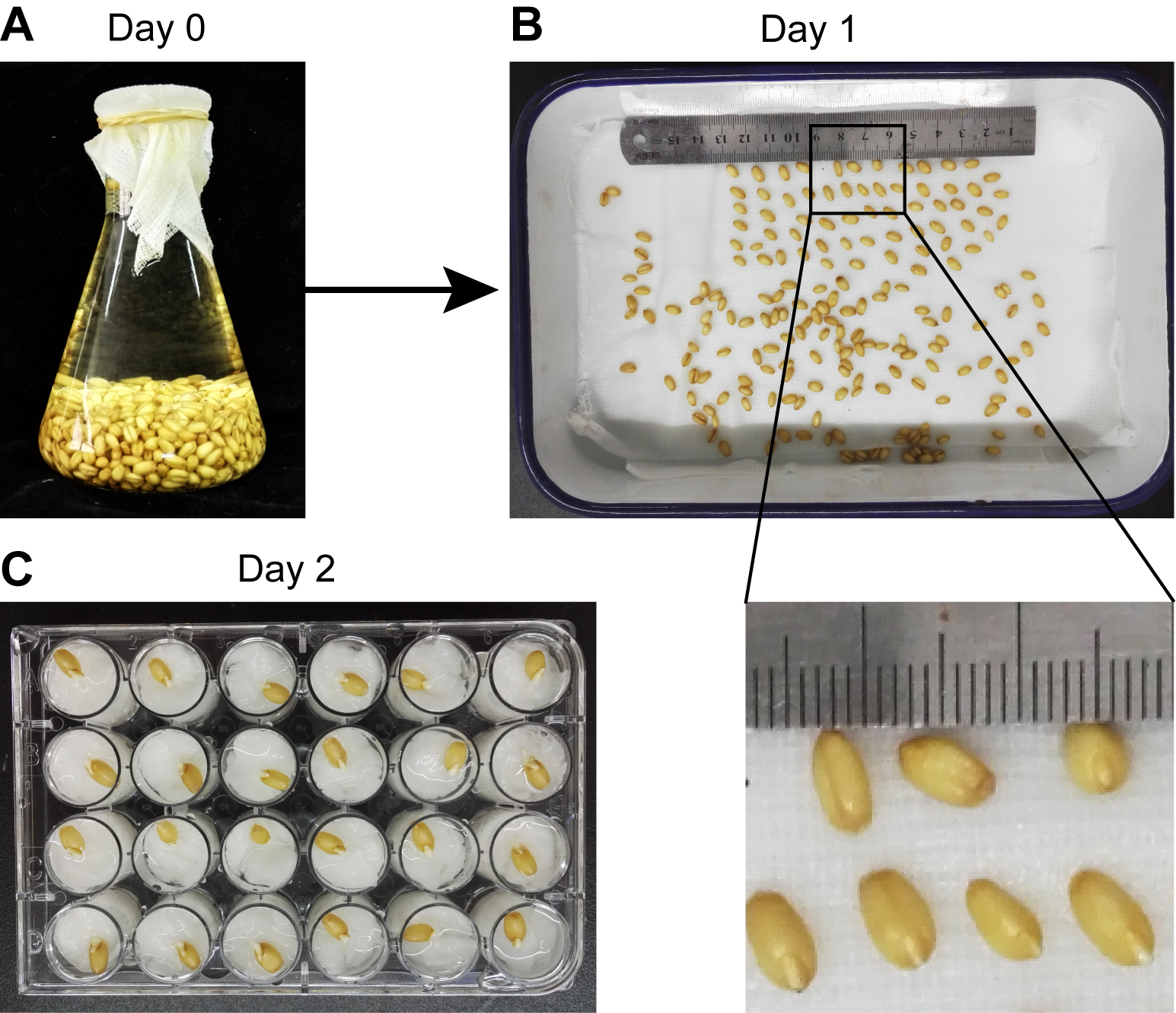
Figure 1. Germination of wheat seeds. Wheat seeds were rinsed in flask with water (A) for one night and then transferred to an enamel tray with wet gauze (B). The germinated seeds were transplanted to 24-well cell culture plates (C). The ruler has scale in mm.
- Soak the wheat seeds at room temperature in a 500 ml flask, and rinse with running water overnight (Figure 1A).
- Preparation of F. graminearum conidia suspension
- From a stock culture, pick a small quantity of F. graminearum mycelia using a sterile toothpick, and transfer to a fresh V8 agar plate. Incubate the culture in 25 °C growth chamber for 3-5 days.
- Scrape the V8 agar plate, now full of aerial hyphae with a sterilized tweezer, and pick up some pieces. Transfer these pieces into 100 ml sterilized mung bean liquid medium, then incubate in a constant temperature shaker at 25 °C and 150 rpm for 3-5 days.
- Filter the mung bean liquid medium culture, then collect the filtered liquid medium into a sterile 250 ml centrifuge bottle. Centrifuge at 7,500 x g for 10 min at room temperature.
- Discard the supernatant and re-suspend the conidia pellet in 1 ml sterile water by pipetting, then transfer the conidia suspension into a 1.5 ml sterile centrifuge tube. Wash the pellet three times using sterile water.
- Resuspend the conidia pellet in sterile water and adjust the concentration to 106 conidia/ml.
Note: The suspension should be used for inoculation within 2 h. Resuspend the conidia just before inoculation.
- From a stock culture, pick a small quantity of F. graminearum mycelia using a sterile toothpick, and transfer to a fresh V8 agar plate. Incubate the culture in 25 °C growth chamber for 3-5 days.
- Wheat coleoptile inoculation
- Cut off the top 1-2 mm of coleoptiles of three-day-old wheat seedlings and add 2 μl F. graminearum wild-type or mutant suspension (106 conidia/ml) to the top of remaining seedling (Figure 2B and Video 1). For mock-inoculation controls, add 2 μl sterile water to the wounded sites.
Note: Check the status of seedlings before inoculation. The coleoptiles of the seedlings ready for inoculation should have not been ruptured, see Figure 2A.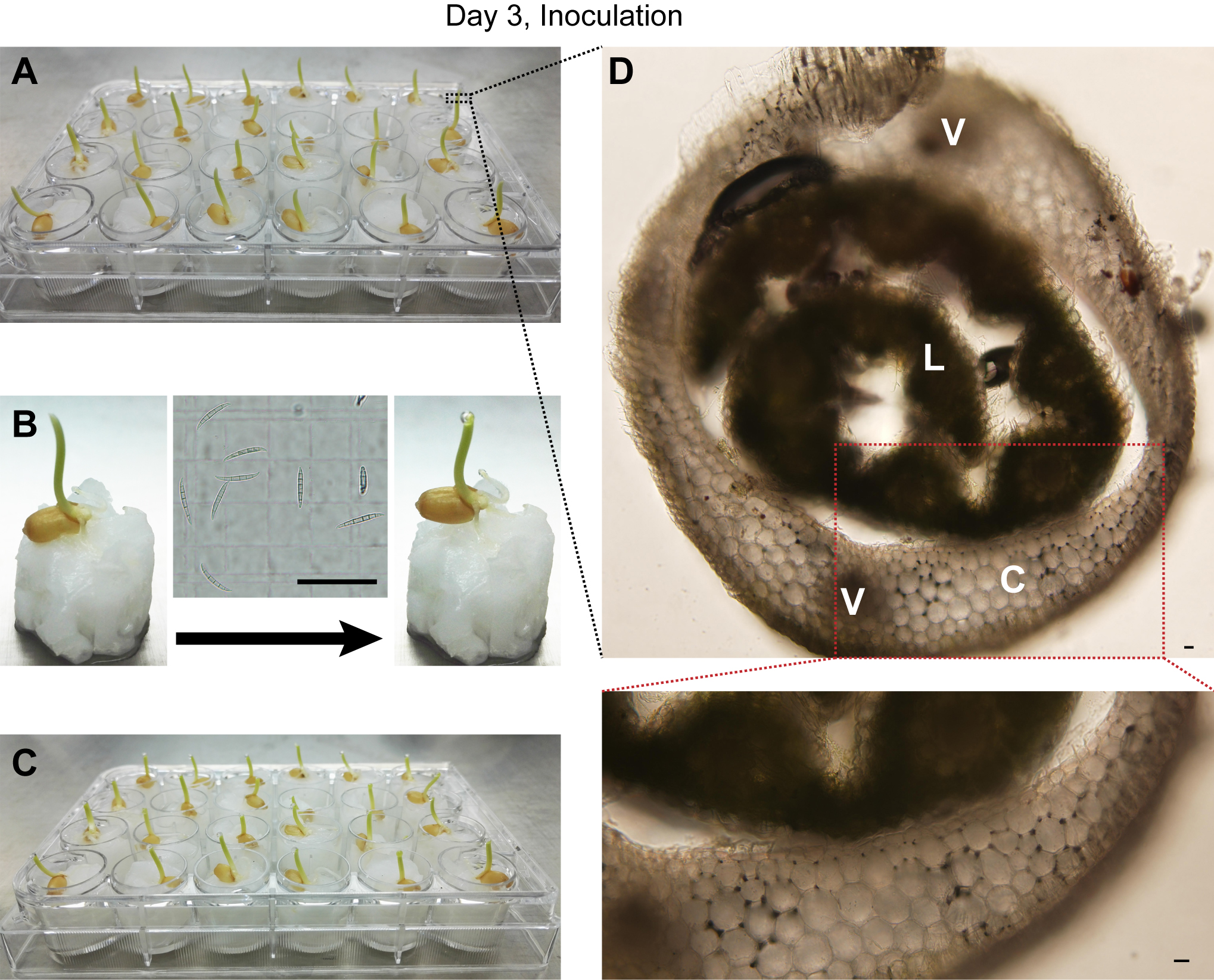
Figure 2. Inoculation of wheat seedlings with conidia suspension. A. Wheat seedlings growing at day 3; B. Process for wheat seedling inoculation; C. Inoculated wheat seedlings; D. Cross section micrograph of wheat seedling. The image inserted in the middle of (B) shows microscopic examination of the conidia suspension on a hemocytometer just before inoculation. C: coleoptile; L: leaf; V: vascular bundle. Scale bars = 20 μm.Video 1. Process for wheat seedling inoculation - Grow the inoculated plants in a growth chamber for another 7 days under controlled environment conditions at 25 °C with a 12 h light/12 h dark photoperiod and 95% relative humidity. The infection progress of fluorescently-tagged F. graminearum strains can be tracked under microscopy (step C4).
Note: The lesions caused by F. graminearum are inconspicuous at the early infection stage (e.g., 1 day post inoculation, dpi). At 2-3 dpi, the coleoptiles at the inoculation sites are turning dark brown and there are aerial hyphae (Figure 3A).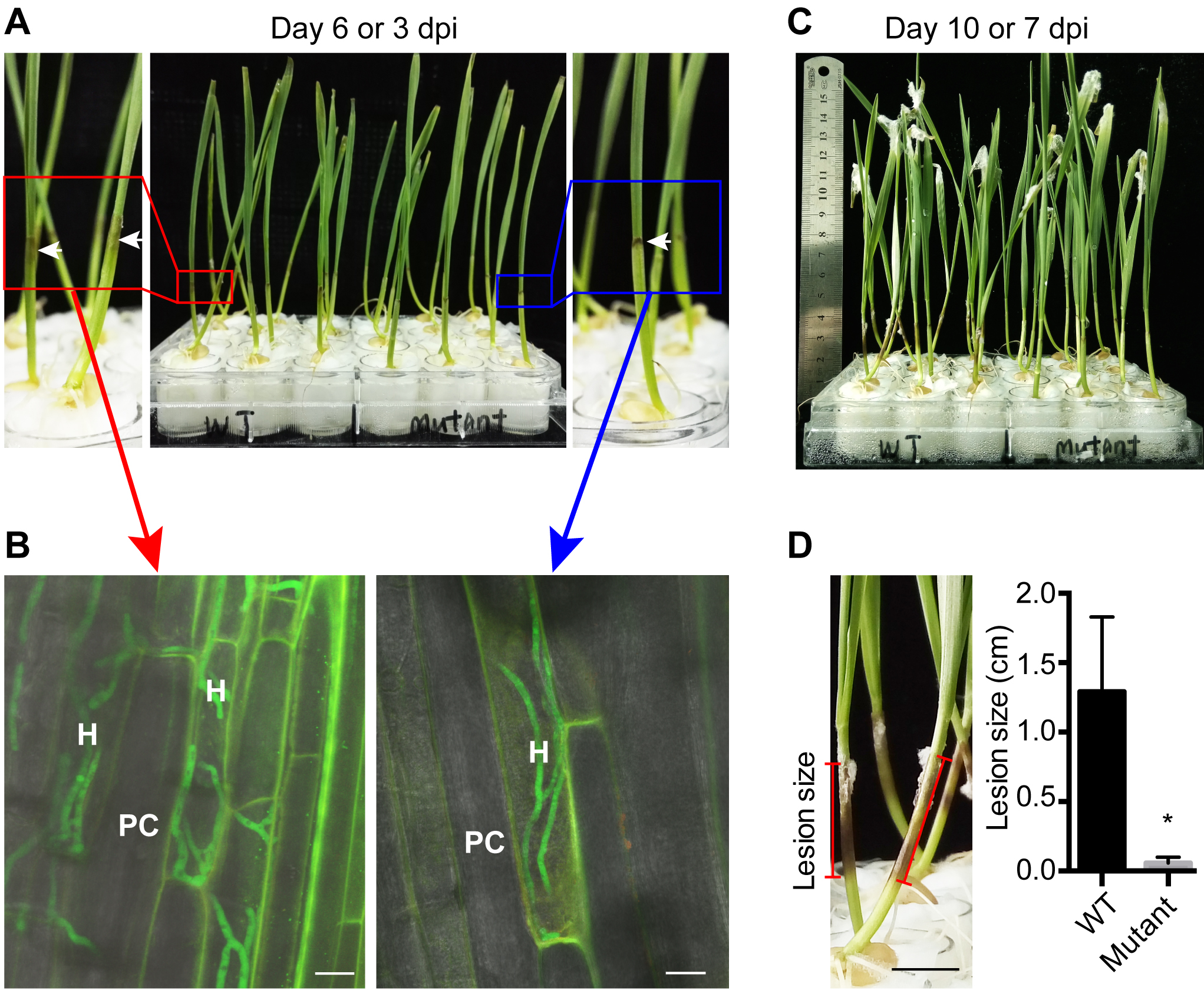
Figure 3. Symptom of F. graminearum infection on wheat seedlings. A. Infected wheat seedlings at 3 dpi (day post inoculation). White arrows show the lesions caused by F. graminearum. B. Growth of F. graminearum hyphae inside wheat coleoptiles. H: hyphae; PC: plant cell. White scale bars represent 20 μm. C. Infected wheat seedlings at 7 dpi; D. Lesion size caused by F. graminearum strains. Black scale bar represents 1 cm. Measurements of one representative experiment are shown. Data are means ± SD (n = 12), *P < 0.001 (significant), Student’s t-test. - Measure the lesion size on coleoptiles of infected wheat seedlings at 7 dpi by photographing them with a ruler as reference (Figure 3C).
Note: Repeat the experiment at least three times, each time use at least 12 seedlings per treatment. - Microscopic observation of wheat coleoptile infection by fluorescently-tagged F. graminearum strains (optional).
The conidia germinated on the coleoptile surface within 10 h-after-inoculation (HAI), and spread quickly inside host tissue from 16 HAI to 64 HAI (Zhang et al., 2012). - Harvest wheat seedlings at given time point after inoculation, and cut off the coleoptiles according to the lesion size.
- Place coleoptiles on a glass slide immersed in sterile water covered by a coverslip.
- Examine coleoptiles using Olympus BX51 microscope or Olympus Fv10i as described in (Zhang et al., 2012; He et al., 2016).
- Chemical treatment (Figure 4) (optional).
- Prepare cotton strips (4 x 2 cm).
- Wrap the cotton strips around the top of the coleoptiles of 1 dpi wheat seedlings (Video 2).
- Add chemical solutions (amino acid solutions, etc.) to the cotton strips.
- Grow the wheat seedlings in a growth chamber for another 6 days under conditions described in step C2.

Figure 4. Process for chemical treatment on wheat coleoptiles after inoculation. One day after inoculation, seedlings ready for chemical treatment are shown in the top panel. Note that new leaves have protruded from the coleoptile. Prepare cotton strips, and wrap the wound sites of the coleoptiles with cotton strips (middle panel). Then pipette chemical solutions (20-40 μl) to the wrapped cotton strips.Video 2. Wrapping the wheat seedling with cotton strip
- Cut off the top 1-2 mm of coleoptiles of three-day-old wheat seedlings and add 2 μl F. graminearum wild-type or mutant suspension (106 conidia/ml) to the top of remaining seedling (Figure 2B and Video 1). For mock-inoculation controls, add 2 μl sterile water to the wounded sites.
Data analysis
The longitudinal length of brown lesions on wheat coleoptiles was measured as the lesion size (Figure 5) at the indicated time using ImageJ. Open the photo taken at 7dpi in ImageJ and choose straight line option to measure 1 cm of ruler in the photo as scale, and set the length as 1 cm. Then measure the length of brown lesions (Figure 5A) and export measurements to excel sheet (Figure 5B). Student’s t-test or one-way ANOVA was used to analyze the virulence with Microsoft Excel (Figure 5C). The diminished lesion size is interpreted as reduced virulence of F. graminearum on wheat coleoptiles (Figure 5D).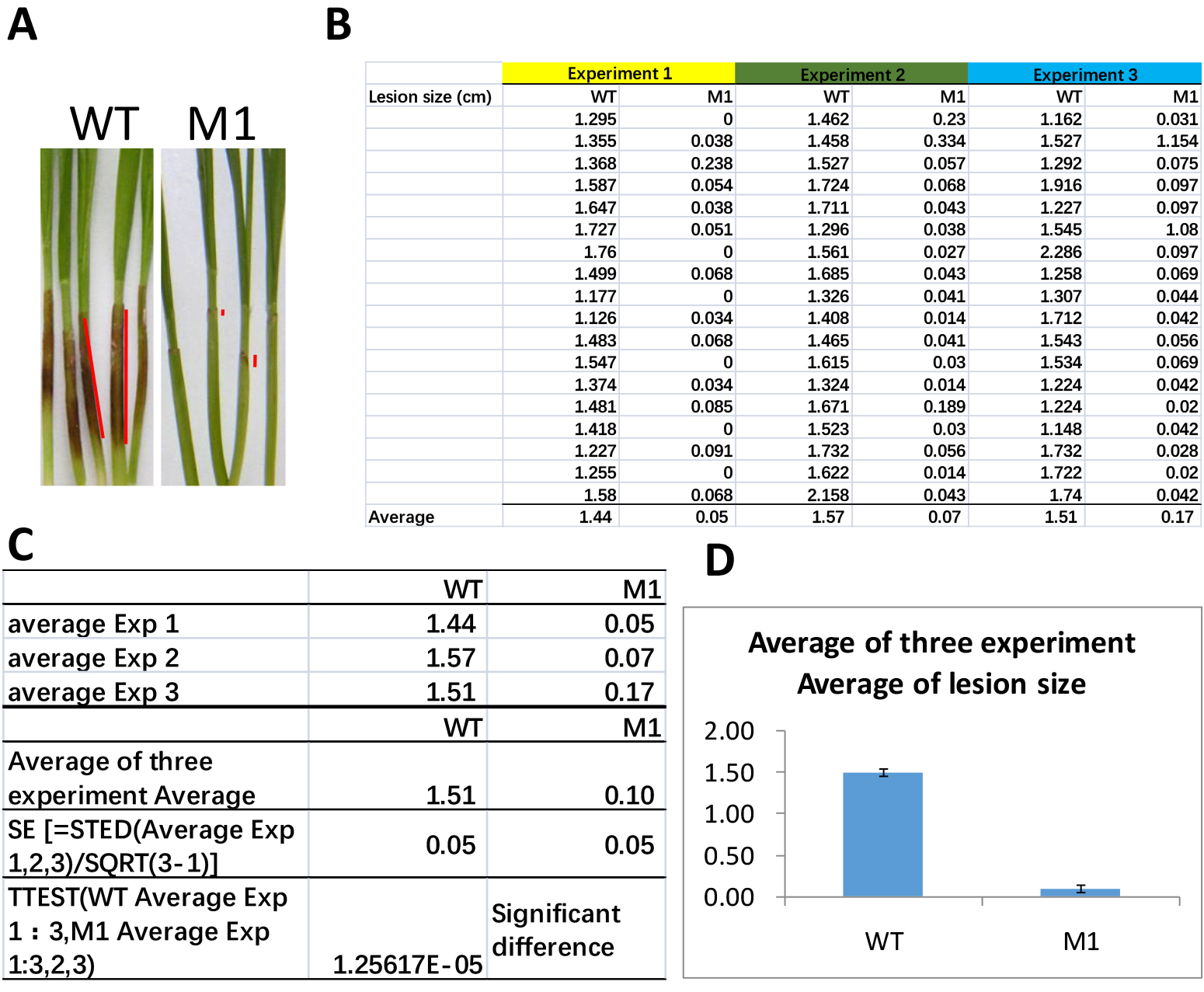
Figure 5. Lesion size measurements and statistical analysis. A. Representative image of seedlings after 7 days inoculation of wild-type (WT) or mutant (M1) strains of F. graminearum. Red lines indicate the ImageJ straight line tool measuring the lesion size. B. The sample data sets of lesion sizes of WT and M1 from three independent experiments in excel. C. Calculation of averages of experimental average lesion sizes and standard errors (SE), and perform t-test analysis. D. Results are charted in bar graph.
Notes
In step C2, it is important to maintain a high humidity environment during the infection progress of F. graminearum.
Recipes
- V8 juice agar medium (1L)
168 ml V8 vegetable juice
1 g CaCO3
15 g agar powder
Autoclave at 121 °C for 20 min - Mung bean liquid medium (1 L)
- 40 g mung beans (Vignaradiata) (dried, available at grocery stores or supermarket)
- Put mung beans in boiling water, then boil for about 10 min and cool to room temperature
- Filter through gauze and discard the bean residue
- Add up to 1 L with distilled water
- Autoclave at 121 °C for 20 min
Acknowledgments
We thank Dr. Sheila McCormick for editing this protocol. This protocol was modified from previous inoculation method Wu et al., 2005 and Zhang et al., 2012. The research in the Tang lab was supported by the Ministry of Agriculture of China (Grant 2016ZX08009-003),the National Key Research and Development Program of China (Grant 2016YFD0100600), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB11020500).
References
- Bai, G. and Shaner, G. (2004). Management and resistance in wheat and barley to fusarium head blight. Annu Rev Phytopathol 42: 135-161.
- Blümke, A., Falter, C., Herrfurth, C., Sode, B., Bode, R., Schafer, W., Feussner, I. and Voigt, C. A. (2014). Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity-related callose formation during wheat head infection. Plant Physiol 165(1): 346-358.
- Dal Bello, G., Mónaco, C. and Simón, M. (2002). Biological control of seedling blight of wheat caused by Fusarium graminearum with beneficial rhizosphere microorganisms. World J Microbiol Biotechnol 18(7): 627-636.
- He, J., Yuan, T.L. and Tang, W.H. (2016). Fusarium graminearum maize stalk infection assay and microscopic observation protocol. Bio Protoc 6(23): e2034.
- Jia, L. J. and Tang, W.H. (2015). The omics era of Fusariumgraminearum: opportunities and challenges. New Phytol 207:1-3
- Kazan, K., Gardiner, D. M. and Manners, J. M. (2012). On the trail of a cereal killer: recent advances in Fusarium graminearum pathogenomics and host resistance. Mol Plant Pathol 13(4): 399-413.
- Proctor, R. H., Hohn, T. M. and McCormick, S. P. (1995). Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact 8(4): 593-601.
- Rawat, N., Pumphrey, M. O., Liu, S., Zhang, X., Tiwari, V. K., Ando, K., Trick, H. N., Bockus, W. W., Akhunov, E., Anderson, J. A. and Gill, B. S. (2016). Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat Genet 48(12): 1576-1580.
- van Eeuwijk, F. A., Mesterhazy, A., Kling, C. I., Ruckenbauer, P., Saur, L., Burstmayr, H., Lemmens, M., Keizer, L. C., Maurin, N. and Snijders, C. H. (1995). Assessing non-specificity of resistance in wheat to head blight caused by inoculation with European strains of Fusarium culmorum, F. graminearum and F. nivale using a multiplicative model for interaction. Theor Appl Genet 90(2): 221-228.
- Wu, A. B., Li, H. P., Zhao, C. S. and Liao, Y. C. (2005). Comparative pathogenicity of Fusarium graminearum isolates from China revealed by wheat coleoptile and floret inoculations. Mycopathologia 160(1): 75-83.
- Zhang, X. W., Jia, L. J., Zhang, Y., Jiang, G., Li, X., Zhang, D. and Tang, W. H. (2012). In planta stage-specific fungal gene profiling elucidates the molecular strategies of Fusarium graminearum growing inside wheat coleoptiles. Plant Cell 24(12): 5159-5176.
- Zhang, Y., He, J., Jia, L. J., Yuan, T. L., Zhang, D., Guo, Y., Wang, Y. and Tang, W. H. (2016). Cellular tracking and gene profiling of Fusarium graminearum during maize stalk rot disease development elucidates its strategies in confronting phosphorus limitation in the host apoplast. PLoS Pathog 12(3): e1005485.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jia, L., Wang, W. and Tang, W. (2017). Wheat Coleoptile Inoculation by Fusarium graminearum for Large-scale Phenotypic Analysis. Bio-protocol 7(15): e2439. DOI: 10.21769/BioProtoc.2439.
Category
Plant Science > Plant immunity > Disease bioassay
Microbiology > Microbe-host interactions > Fungus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link