- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Purification of FLAG-tagged Secreted Proteins from Mammalian Cells
Published: Vol 7, Iss 15, Aug 5, 2017 DOI: 10.21769/BioProtoc.2430 Views: 11678
Reviewed by: Jyotiska ChaudhuriLiang LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

ZnCl2 Precipitation-Assisted Sample Preparation for Proteomic Analysis
Qiqing He [...] Fuchu He
Jul 20, 2025 2726 Views

Protocol for the Preparation of a Recombinant Treacle Fragment for Liquid–Liquid Phase Separation (LLPS) Assays
Nadezhda V. Petrova [...] Artem K. Velichko
Sep 20, 2025 1813 Views

Optimized Secretome Sample Preparation From High Volume Cell Culture Media for LC–MS/MS Proteomic Analysis
Basil Baby Mattamana [...] Peter Allen Faull
Dec 20, 2025 1228 Views
Abstract
This protocol describes a method for purifying glycosylated FLAG-tagged secreted proteins with disulfide bonds from mammalian cells. The purified products can be used for various applications, such as ligand binding assays.
Keywords: Secreted proteinBackground
E. coli is one of the organisms of choice for production of recombinant proteins from both prokaryotes and eukaryotes. The major advantages of bacterial expression systems are high productivity and low cost. However, mature proteins are essential for functional analyses (e.g., ligand binding assays). Most secreted eukaryotic proteins undergo post-translational modification by covalent glycan linkage and formation of disulfide bonds in the endoplasmic reticulum. These covalent modifications are essential for the general stability and folding of the secreted mature proteins. Therefore, the best method for production of mammalian secreted proteins is the use of a mammalian host to ensure the production of recombinant proteins that have undergone proper post-translational modifications. In the following protocol, we have described the detailed procedure for purification of secreted proteins from mammalian cells. This protocol is for production of FLAG-tagged proteins, but should be applicable to that of other tagged (e.g., His-tag) proteins as well.
Materials and Reagents
- Pipette tips (VWR)
- 12-well plate (Corning, Falcon®, catalog number: 353043 )
- 100 mm tissue culture dishes (Corning, Falcon®, catalog number: 353803 )
- Amicon Ultra 15 filtration units 3K (EMD Millipore, catalog number: UFC900308 )
- 1.5 ml tubes (VWR, catalog number: 89000-028 )
- 50 ml Falcon tubes (Corning, Falcon®, catalog number: 352070 )
- 1 ml syringes (Terumo Medical, catalog number: SS-01T )
- 30 G needles (BD, catalog number: 305128 )
- Flp-In T-rex 293 cell line (Thermo Fisher Scientific, InvitrogenTM, catalog number: R78007 ) (Optional)
- pcDNA5 FRT TO (Thermo Fisher Scientific, InvitrogenTM, catalog number: V652020 ) (Optional)
- Hygromycin B (NACALAI TESQUE, catalog number: 07296-11 ) (Optional)
- Blasticidin (Wako Pure Chemical Industries, catalog number: 029-18701 ) (Optional)
- Doxycycline (Takara Bio, Clontech, catalog number: 631311 ) (Optional)
- Dulbecco’s modified eagle’s medium (DMEM) (Sigma-Aldrich, catalog number: D6546 )
- Fetal bovine serum (FBS) (Biosera, catalog number: FB-1345/500 )
- Anti-Flag M2 agarose beads (Sigma-Aldrich, catalog number: A2220 )
- Penicillin-streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15070063 )
- Potassium acetate (KAc) (Sigma-Aldrich, catalog number: 236497 )
- HEPES (Sigma-Aldrich, catalog number: H3375 )
- Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266 )
- 3xFlag peptide (Sigma-Aldrich, catalog number: F4799 )
- Phosphate buffered saline (PBS)
- Growth medium (see Recipes)
- Wash buffer (see Recipes)
- Elution buffer (see Recipes)
Equipment
- Pipettes (e.g., Gilson)
- 37 °C, 5% CO2 incubator (e.g., Panasonic Biomedical, catalog number: MCO-170AICUV-PE )
- Tube rotator located in cold room (e.g., Fisher Scientific, catalog number: 88-861-049 )
- Centrifuge (e.g., Eppendorf, model: 5804 )
- Cell culture microscope (e.g., Carl Zeiss, Vienna, Austria)
Procedure
- Generation of stable cell lines using the Flp-In T-rex 293 cell line (Figure 1)
Note: The Flp-In T-rex 293 cell line is suitable for inducible, stable, and homogenous high expression of your 3xFLAG-tagged protein of interest. Transiently transfected cell line can be used as long as the transfection efficiency (determined by immunofluorescence) is more than 50%.
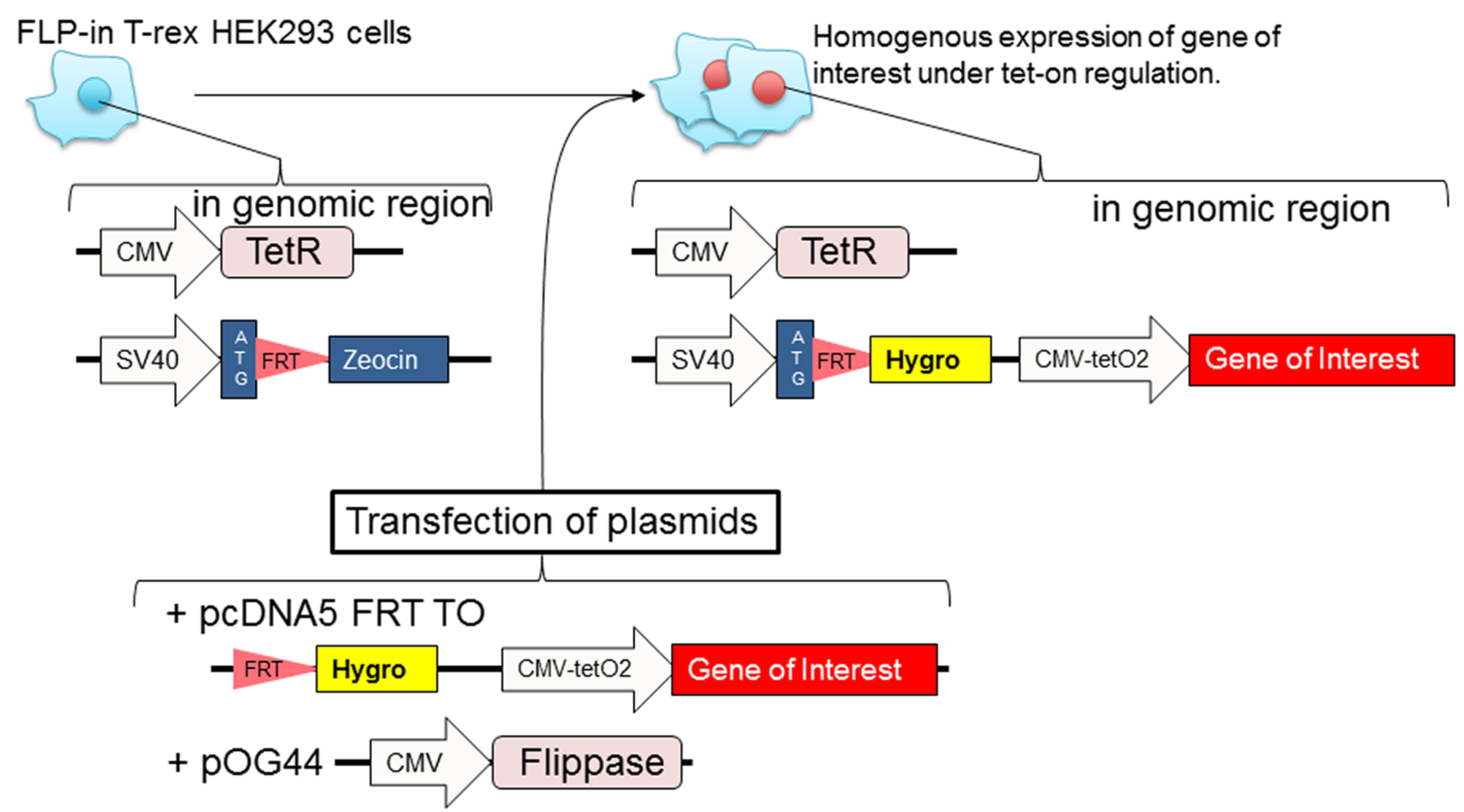
Figure 1. Diagram of how to prepare stable Flp-in T-rex 293 cell lines. Transfect Flp-in T-rex 293 cells with plasmids (pcDNA5 FRT TO with gene of interest and pOG44 that contains a gene for flippase). The following day, the cells are cultured with hygromycin B to select cells that have incorporated the gene of interest at FRT site of genomic region. The polyclonal stable cell lines are homogeneous expression.
- Seed 1 x 105 Flp-In T-rex 293 cells in each well of a 12-well plate.
- Incubate overnight when cells have reached approximately 50% of confluency, and transfect Flp-In T-rex 293 cells with pcDNA5 FRT TO containing your FLAG-tagged gene of interest and pOG44 plasmids.
- After 4-5 days of transfection, treat cells with 100 μg/ml hygromycin B and 15 μg/ml blasticidin for approximately 2 weeks.
- Seed 1 x 105 Flp-In T-rex 293 cells in each well of a 12-well plate.
- Purification of FLAG-tagged protein
- Grow five 100 mm dishes of Flp-In T-rex 293 cells expressing the 3xFLAG-tagged protein in growth medium (see Recipes) supplemented with 10 μg/ml doxycycline for 1 day until cells are approximately 80% confluent.
- Change cell culture media to 10 ml DMEM containing 5% FBS with 10 μg/ml doxycycline and culture for 2 days.
Note: 10% FBS medium may clog an Amicon centrifuge filter during the concentration step.
- Harvest the cell culture media (conditioned media) from all five 100 mm dishes of step B2 into a 50 ml Falcon tube.
- Centrifuge the conditioned media at 2,000 x g for 5 min at 4 °C.
- Transfer the supernatant into an Amicon centrifuge filter unit. Spin at 4,000 x g for 30-40 min.
- Repeat step B5 until the retention volume is less than 2 ml.
- Transfer the concentrated conditioned media into two 1.5 ml tubes (1 ml per tube).
- Add 100 μl pre-washed 50% FLAG M2 beads slurry to each tube.
- Incubate the beads at least 2 h while rotating at 4 °C.
- Wash the beads with 0.5 ml cold wash buffer (see Recipes) 4 times by spinning at 100 x g for 1 min. After the last wash, remove as much buffer as possible with minimal disruption of the beads using syringe needles.
- Incubate the beads with 0.2 ml elution buffer (see Recipes) for 1 h at room temperature and collect the purified FLAG-tagged protein after centrifugation.
- Grow five 100 mm dishes of Flp-In T-rex 293 cells expressing the 3xFLAG-tagged protein in growth medium (see Recipes) supplemented with 10 μg/ml doxycycline for 1 day until cells are approximately 80% confluent.
Recipes
- Growth medium (560.5 ml)
500 ml DMEM
55 ml 10% FBS
5.5 ml 1% penicillin-streptomycin
- Wash buffer (50 ml)
5.5 ml 1 M KAc (110 mM)
1 ml 1 M HEPES (pH 7.4) (20 mM)
100 μl 1 M MgCl2 (2 mM)
Make up to 50 ml with Millipore water
- Elution buffer (210 μl)
10 μl 5 mg/ml 3xFlag peptide (0.25 mg/ml)
200 μl 1x PBS
Acknowledgments
This protocol was adapted from Chen et al. (2017). This work was supported by JSPS KAKENHI (Young Scientists A) Grant Number 16H06167, MEXT KAKENHI (Scientific Research on Innovative Areas) Grant Number 16H01194 (to E. I.); a European Molecular Biology Organization Fellowship (to C.C.); the Medical Research Council, UK; and the European Research Council (Advanced Grant 269058) grant to M.d.B.
References
- Chen, C., Itakura, E., Nelson, G. M., Sheng, M., Laurent, P., Fenk, L. A., Butcher, R. A., Hegde, R. S. and de Bono, M. (2017). IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature 542(7639): 43-48.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Itakura, E., Chen, C. and de Bono, M. (2017). Purification of FLAG-tagged Secreted Proteins from Mammalian Cells. Bio-protocol 7(15): e2430. DOI: 10.21769/BioProtoc.2430.
Category
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









