- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel Mouse Skin Graft Model of Vascular Tumors Driven by Akt1
Published: Vol 7, Iss 13, Jul 5, 2017 DOI: 10.21769/BioProtoc.2369 Views: 11462
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Optimized Protocol for Simultaneous Propagation of Patient-derived Organoids and Matching CAFs
Jenny M. Högström and Taru Muranen
Jan 20, 2025 4427 Views

Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle
Catalina Matias [...] Jeffrey J. Brault
Apr 5, 2025 2900 Views

A Novel Optimized Silver Nitrate Staining Method for Visualizing and Quantifying the Osteocyte Lacuno-Canalicular System (LCS)
Jinlian Wu [...] Libo Wang
Apr 20, 2025 1518 Views
Abstract
To investigate whether endothelial Akt1 activation is sufficient to induce vascular tumor formation in the skin, we have developed a skin graft model in which a skin fragment from transgenic donor mice with inducible and endothelial cell-specific overexpression of activated Akt1 (myrAkt1) is grafted into the skin of wild type recipient mice. The donor skin successfully engrafts after two weeks and, more importantly, vascular tumor develops at the site of transgenic skin graft when myrAkt1 expression is turned on. This skin graft model is a novel approach to investigate the biological impact of a key signal transduction molecule in a temporal, localized and organ-specific manner.
Keywords: Vascular tumorsBackground
Our research focuses on investigating the role of Akt1 in vascular tumor development. To determine whether the activation of Akt1 in endothelial cells is sufficient to drive de novo vascular tumor formation, we have developed and published a skin graft model of vascular tumor (Phung et al., 2015). We developed the current protocol of grafting skin from transgenic mice onto the skin of wild type mice because this is a way to study the localized and skin-specific effects of the overexpression of constitutively active Akt1 in the vasculature. We have observed that overexpression of Akt1 in the systemic vasculature leads to generalized edema in the lungs and skin, resulting in premature death of transgenic animals within days due to massive pulmonary edema. A procedure to graft transgenic skin onto wild type host mouse allows one to examine the long-term effects of Akt1 overexpression in only the transgenic skin vasculature without the lethality associated with systemic Akt1 overexpression. In our studies, we are particularly interested in investigating the long-term effects of Akt1 in the skin vasculature on the development of vascular tumors based on the hypothesis that hyperactivation of Akt1 in endothelial cells is sufficient to induce vascular tumor formation, thus demonstrating the endothelial cell-autonomous effects of Akt1 in these tumors.
In the model, skin from double transgenic mice with inducible expression of activated Akt1 (myrAkt1) in endothelial cells is grafted onto the back of immunodeficient nu/nu recipient mice. Since myrAkt1 expression is inducible, we can turn off myrAkt1 expression at will by giving the animals tetracycline in their drinking water, and we can turn on myrAkt1 expression by removing tetracycline from the water. A detailed description of the myrAkt1 double transgenic mice has been published (Sun et al., 2005). A schematic of the construction of these mice is shown (Figure 1). Using the skin graft model, we were able to demonstrate that expression of myrAkt1 in endothelial cells is sufficient for the development of vascular tumor. The procedural steps of this model are described below. 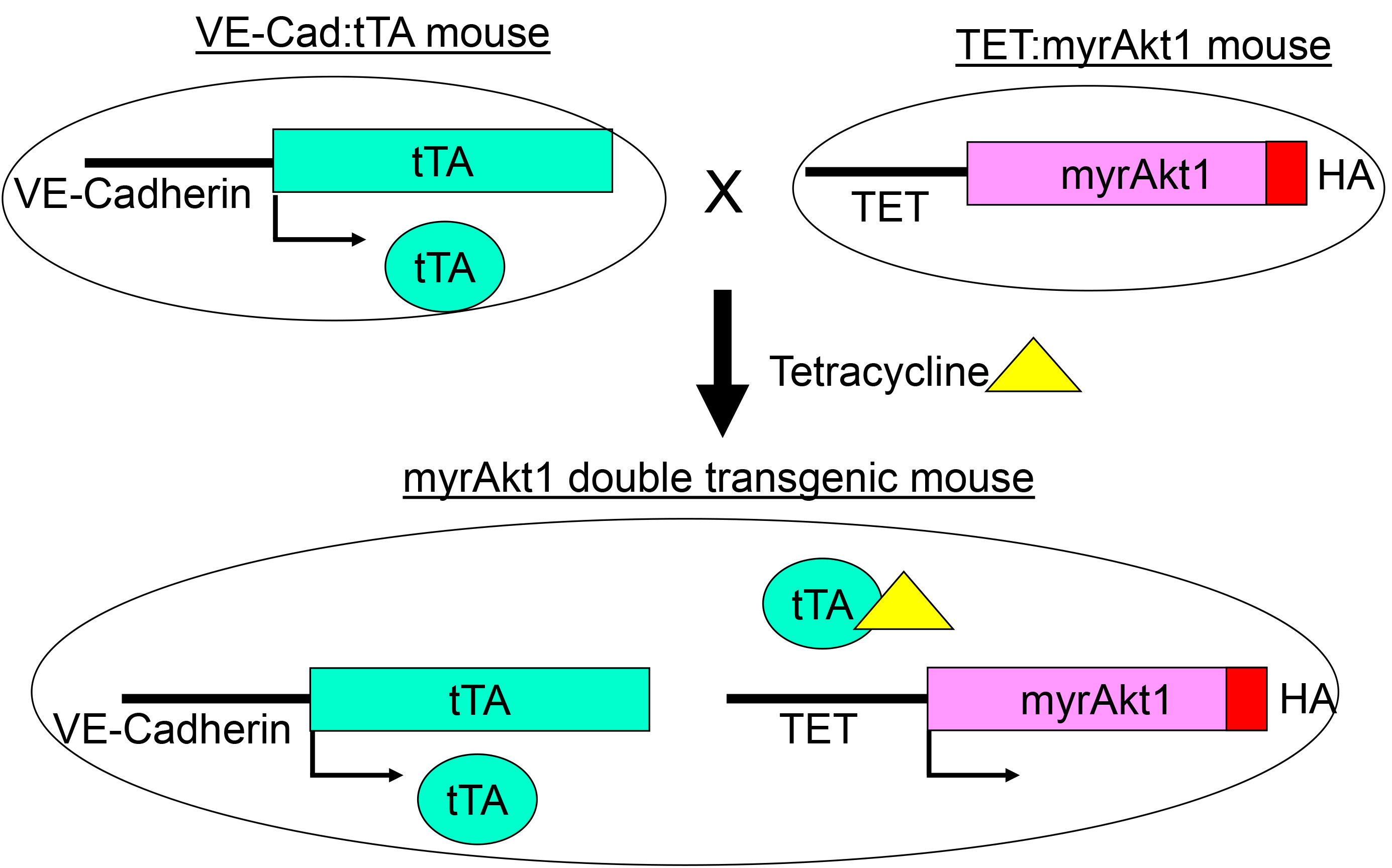
Figure 1. myrAkt1 double transgenic mouse model. The VE-Cad:tTA mouse line contains the VE-cadherin promoter cloned upstream of the tetracycline-regulated transcriptional activator (tTA) gene. VE-cadherin promoter is mainly active in endothelial cells. The TET:myrAkt1 line carries a constitutively activated Akt1 with an HA tag (myrAk1) under the control of the tetracycline responsive promoter (TET). To suppress myrAkt1 expression in the double transgenic mice, 1.5 mg/ml tetracycline with 5% sucrose is given to the mice in their drinking water. To turn on myrAkt1 expression, the mice are given pure water without tetracycline. In this system, double transgenic mice express myrAkt1 in endothelial cells, and the expression of myrAkt1 can be regulated with tetracycline (Reference: Sun JF, Phung T, Shiojima I, Felske T, Upalakalin JN, Feng D, Kornaga T, Dor T, Dvorak AM, Walsh K, Benjamin LE. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci USA 2005; 02: 128-33).
Materials and Reagents
- Sterile surgical gloves
- 10 mm skin punch biopsy instrument (Acu-Punch Biopsy Punch 10 mm) (Acuderm, catalog number: P1025 ) (Figure 2)

Figure 2. A 10-mm punch biopsy instrument - Ethilon non-absorbable nylon suture, 5-0 PC-3 (Ethicon, catalog number: 1965G )
- Sterile surgical towel drape (Steri-Drape) (3M, catalog number: 1010 )
- Sterile surgical gauze (4 x 4 Gauze 8 Ply) (COVIDIEN, DermaceaTM, catalog number: 441001 )
- Alcohol pads (WebCol Alcohol Prep) (COVIDIEN, catalog number: 6818 )
- Donor mice (females, 6-8 weeks old transgenic mice, see reference for the generation of transgenic mice: Sun JF, Phung T, Shiojima I, Felske T, Upalakalin JN, Feng D, Kornaga T, Dor T, Dvorak AM, Walsh K, Benjamin LE. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci U S A 2005; 102: 128-33)
- Recipient mice (females, 6-8 weeks old nu/nu mice from Charles River Laboratories, catalog number: 490 )
- Isoflurane (Sigma-Aldrich, catalog number: CDS019936 )
- Phosphate buffered saline (PBS), 1x sterile solution (AMRESCO, catalog number: K812 )
Equipment
- Needle holder (Halsey serrated jaws) (Roboz Surgical Instrument, catalog number: RS-7841 )
- Fine single-toothed forceps (Micro dissecting tweezers Pattern 7) (Roboz Surgical Instrument, catalog number: RS-5047 )
- Surgical scissors (Micro dissecting scissors) (Roboz Surgical Instrument, catalog number: RS-5910 )
- Electric hair shaver (Wahl Battery Operated Compact Travel Trimmer)
- Warming plate (Ala Science, catalog number: TCW220x120 )
Procedure
Important: Appropriate Institutional Animal Care and Use Committee (IACCU) approval must be obtained prior to performing the skin graft procedure in animals.
- Anesthetize donor and recipient mice with isoflurane anesthetic gas. The donor animal is typically sedated for 5-10 min while the punch biopsy and removal of donor skin is performed. The recipient animal is typically sedated for 20-30 min while the two skin grafts are sutured in place. Animals are adequately sedated when they do not display a response to pinching of the foot pad.
- For the donor mouse, chose a skin area from where the skin will be taken. In our study, we selected the back skin because the skin would be grafted at the same anatomic site on the back of the recipient mouse.
- Shave the hair over the skin area from where the skin graft will be taken. Be careful to avoid scratching or injuring the skin surface (Figure 3 and Video 1).

Figure 3. Procedure for removing a punch of skin from donor mouse. A-B. Shave the hair over the skin area from where the skin graft will be taken. C. Hold the skin stretched slightly and position the 10-mm punch instrument vertically on top of the stretched skin. D. Gently rotate the punch instrument until the punch cuts through the skin. E. Once the punch cuts through the skin, remove the punch. F-G. Cut out the piece of punched skin and underlying subcutaneous tissue, and place the skin on a piece of sterile gauze soaked in sterile phosphate buffered saline to keep the skin moist. A close-up photo of the host skin punch with the skin dermis side up is shown in panel G.Video 1. Skin graft procedure - Place the animal onto a clean surface covered with a sterile surgical drape.
- Clean the shaved skin of the donor animal thoroughly with 70% alcohol wipe pads.
- Hold the skin stretched slightly, and position the 10-mm punch instrument vertically on top of the stretched skin. Gently rotate the punch instrument with a smooth 360° turn motion while applying downward pressure until the punch cuts through the skin (also see video demonstration of the technique by Levitt et al., 2013).
- Once the punch cuts through the skin and reaches its maximum depth of approximately 1-2 mm through the dermis and subcutaneous tissue, remove the punch. Cut out the piece of punched skin and underlying subcutaneous tissue with sterile fine surgical scissors.
- Gently remove the skin fragment with sterile fine forceps. The forceps are sterilized by autoclaving. Please note, when removing the skin, be careful to avoid crushing it by picking up the tissue piece at the edge with fine single-toothed forceps. Save the punched-out skin in order to graft it onto the back of the recipient mouse. Keep the skin graft moist by placing it onto a piece of sterile gauze soaked in 1x sterile phosphate buffered saline (PBS) solution.
- Hemostasis can be achieved by applying gentle pressure over the cut area with a dry sterile surgical gauze. Once this step is completed, the donor animal can be immediately euthanized per approved Institutional Animal Care and Use Committee (IACCU) protocol.
- Repeat the same punch biopsy procedure for the recipient mouse. Punch out a 10-mm circular piece of skin from the back of the recipient mouse. Cut out the skin fragment and the underlying subcutaneous tissue and discard it (Figure 4 and Video 1).
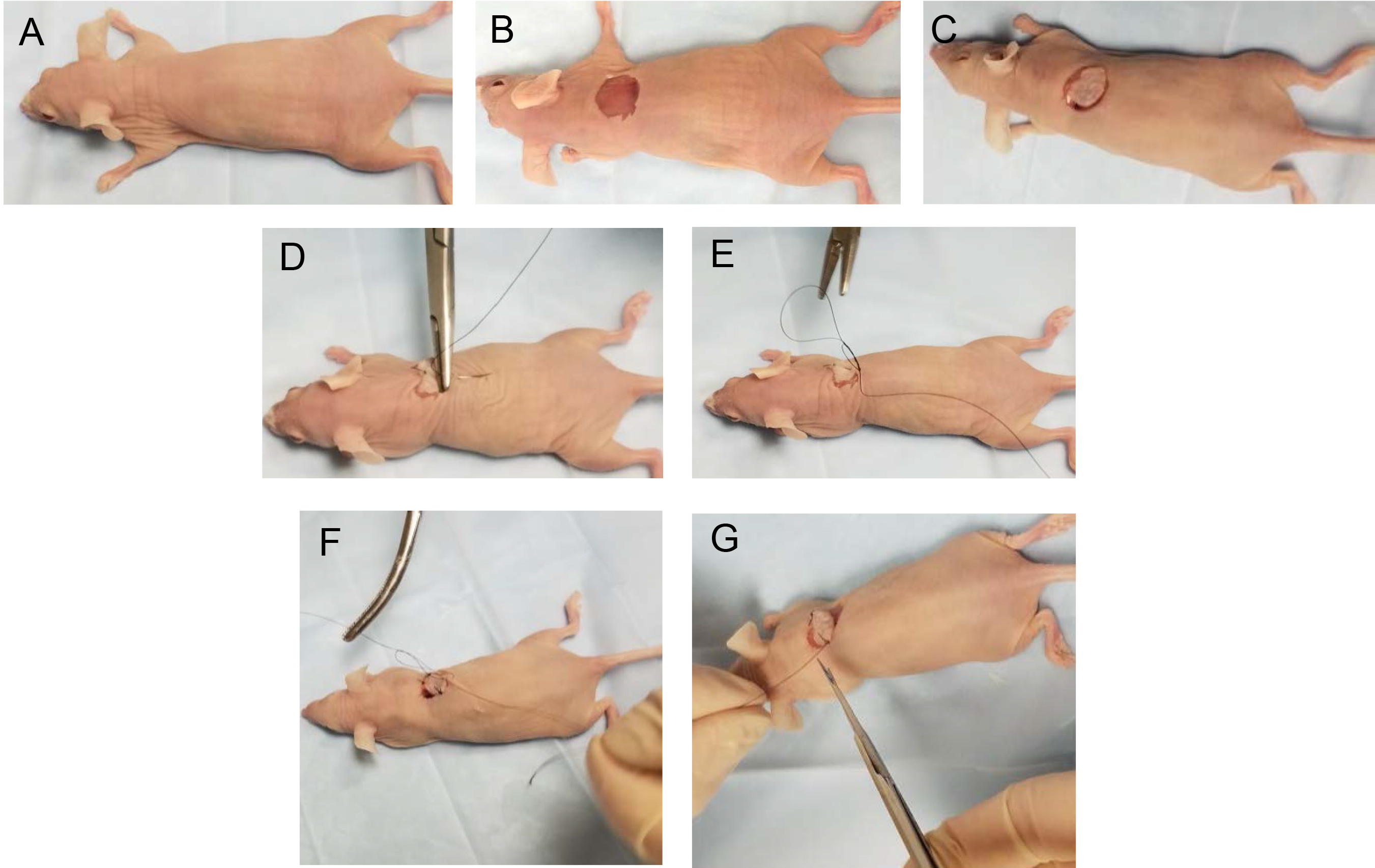
Figure 4. Procedure for suturing donor skin onto recipient mouse. A-B. Remove a piece of skin from the back of the recipient mouse using a punch device as described in Figure 3. C. Take the donor skin fragment and put it into place in the punched out area on recipient mouse. D-G. Suture donor skin fragment into place. - Apply gentle pressure over the cut area with a sterile surgical gauze to stop any bleeding.
- Take the donor skin fragment and put it into place in the punched out area on the back of the recipient mouse.
- To help position the skin graft in place, use the circular skin punch as a clock face. First, place a suture stitch at 12, 3, 6 and 9 o’clock positions. Then add a suture stitch at 1:30, 4:30, 7:30 and 10:30 o’clock positions. Suture the skin fragment in place with non-absorbable nylon suture. Perform double tie knots to secure suture thread in place.
- When completed, clean the sutured area by gently wiping it with a sterile gauze soaked in sterile PBS solution.
- Keep the animal under observation on a warming plate at 37 °C until it is fully awake and moving, which typically takes about 10-15 min. Animal usually awakens and starts moving soon after anesthesia is withdrawn, so there needs to be constant observation until the animal is fully awake. Once the animal is fully awake and can move, it can be placed back into the cage with clean cage bedding, water and food (place a few food pellets on the cage floor so the animal can feed easily during the first 1-2 days post procedure).
- Monitor the animal daily for signs of wound infection and for evidence of healing and wound closure (Figure 5D).
- Sutures can be removed 10-14 days after the surgical procedure, which is approximately the time frame needed for complete skin wound healing.
- Successful skin engraftment is evident as the grafted donor skin becomes pink with hair growth in the skin graft (Figure 5D).
Note: Detailed demonstration of the entire procedure can be viewed in our video that is included with this paper. Additional information on how to perform a skin punch biopsy can also be obtained from the video reference in Levitt et al., 2013.
Data analysis
Using this skin graft model to study the effects of endothelial Akt1 on vascular tumor formation, we have found that overexpression of constitutively activated Akt1 for 4 weeks resulted in the development of red masses at the skin graft sites that measured 0.39 ± 0.14 cm3 in size and had histologic features of a vascular tumor (Figures 5 and 6) (also see Figure 3 in Phung et al., 2015). Immunofluorescence staining of tumor sections shows that tumor vessels express the endothelial marker CD31 (Figure 6D). For immunofluorescence staining, fresh tissues are fixed in cold 4% paraformaldehyde, blocked in 5% goat serum for 1 h, and incubated with anti-CD31 antibody (BD Biosciences MEC13.39) overnight at 4 °C, followed by incubation for 1 h with DylightTM 488-conjugated secondary antibody (Jackson ImmunoResearch Labs). More detailed information on the immunofluorescence staining technique and analysis of skin graft tumors is presented in the original publication (adapted from Phung et al., 2015).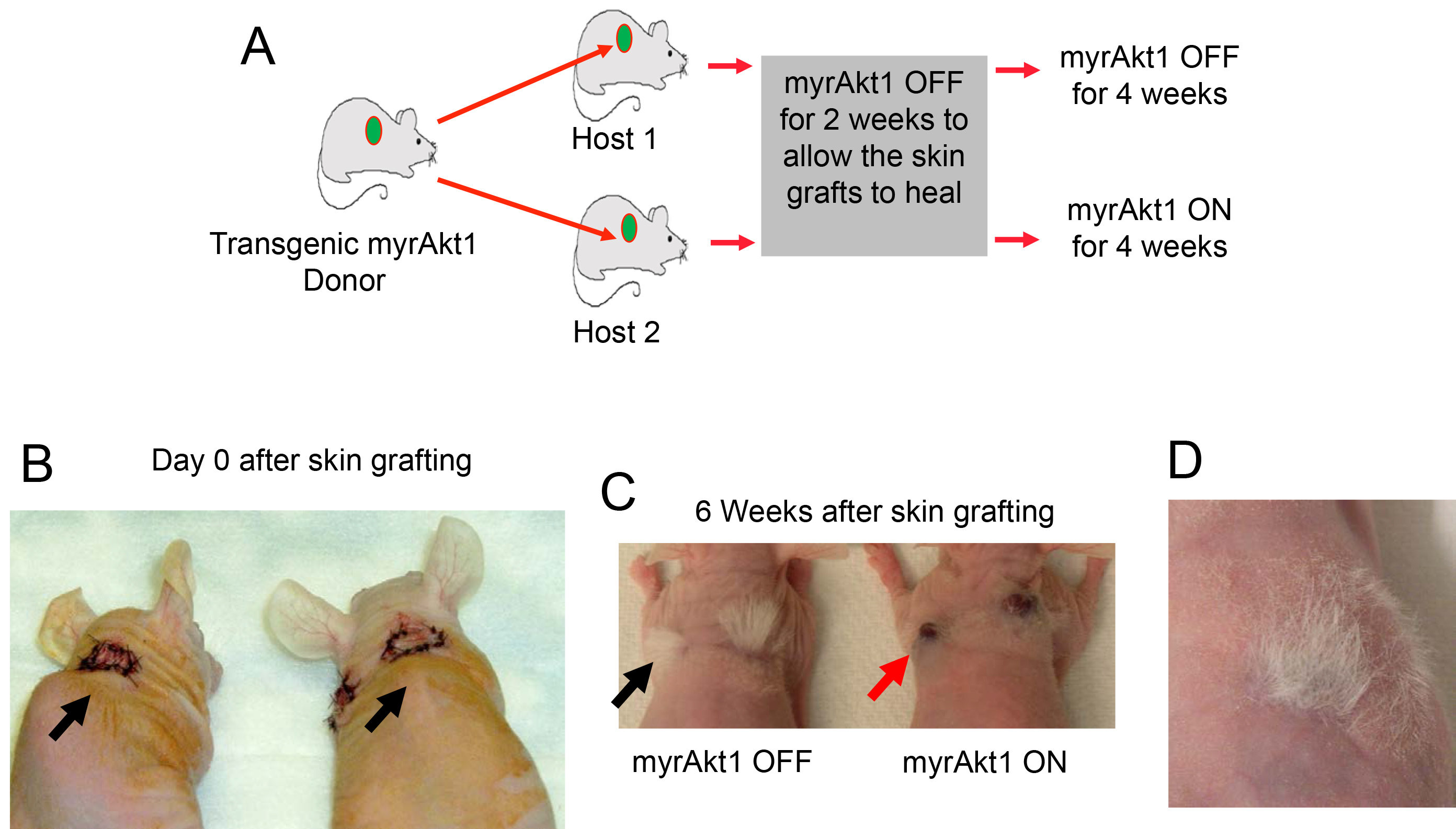
Figure 5. Mouse model of endothelial myrAkt1-driven vascular tumor. A. Schematic of myrAkt1 skin graft model of hemangioma. Transgenic donor mice in FVB background express inducible expression of myristoylated activated Akt1 (myrAkt1) in endothelial cells. Skin from myrAkt1 mice (green circles) was used as skin graft donors in nu/nu recipient mice. 10-mm biopsy punches of skin from donor mice were grafted onto the back skin of host (recipient) mice. Grafts were allowed to heal for 2 weeks, at which time half of the recipient mice were taken off tetracycline (given plain drinking water) to turn on myrAkt1 and the other half were kept on tetracycline to turn off myrAkt1 for 4 weeks. B. Photographs of recipient nu/nu mice showing fresh skin grafts with suture on day 0 (arrows). C. Photographs of mice 6 weeks later showing the presence of successful skin engraftment with hair growth in the skin graft (black arrow indicates tuft of white hair in graft site). There is no tumor mass at the skin graft sites in recipient mouse with myrAkt1 OFF, but there are red tumor masses (see red arrow) in the skin graft sites in recipient mouse with myrAkt1 ON. D. Successful skin engraftment is evident by healing of the donor skin graft and the grafted skin becomes pink with hair growth in the graft site (Reference: Phung, T. L., Du, W., Xue, Q., Ayyaswamy, S., Gerald, D., Antonello, Z., Nhek, S., Perruzzi, C. A., Acevedo, I., Ramanna-Valmiki, R., Rodriguez-Waitkus, P., Enayati, L., Hochman, M. L., Lev, D., Geeganage, S. and Benjamin, L. E. (2015). Akt1 and akt3 exert opposing roles in the regulation of vascular tumor growth. Cancer Res 75(1): 40-50).
Figure 6. Endothelial myrAkt1 activation drives vascular tumor formation in vivo. A. Donor mouse skin engrafted in nu/nu recipient mouse as seen by the presence of tuffs of white hair at the graft site. Vascular tumor development in the skin graft was monitored following myrAkt1 induction. Tumor developed in the skin graft 4 weeks following myrAkt1 induction. Magnification X1. B. Microscopic features of the tumor showing a tumor mass (arrows). C. The tumor consists of numerous blood vessels filled with red blood cells in the dermis (arrows). The vessels have variable sizes and irregular lumen with thin vascular wall. D. The tumor is composed of numerous blood vessels as shown in CD31 immunofluorescence stain (green). Cell nuclei are stained with Hoechst dye (blue).
Notes
In our experience, the skin graft procedure works successfully almost all the time as evident by the successful engraftment of the donor skin. We have not observed animal death or skin infection at the graft site. Because immunodeficient nu/nu recipient mice are used as graft recipients, there is no evidence of graft rejection in our study.
Acknowledgments
This work was supported in part by NIH grants R01 CA106263 and P01 CA09264401 (to L. E. Benjamin), American Heart Association 11BGIA5590018, NIH K08 HL087008, NIH R03 AR063223, American Cancer Society 122019-RSG-12-054-01-CSM, and the Baylor Clinical and Translational Research Program (to T. L. Phung).
References
- Levitt, J., Bernardo, S., Whang, T. (2013). Videos in clinical medicine. How to perform a punch biopsy of the skin. N Engl J Med 369(11): e13.
- Phung, T. L., Du, W., Xue, Q., Ayyaswamy, S., Gerald, D., Antonello, Z., Nhek, S., Perruzzi, C. A., Acevedo, I., Ramanna-Valmiki, R., Rodriguez-Waitkus, P., Enayati, L., Hochman, M. L., Lev, D., Geeganage, S. and Benjamin, L. E. (2015). Akt1 and akt3 exert opposing roles in the regulation of vascular tumor growth. Cancer Res 75(1): 40-50.
- Sun, J. F., Phung, T., Shiojima, I., Felske, T., Upalakalin, J. N., Feng, D., Kornaga, T., Dor, T., Dvorak, A.M., Walsh, K. and Benjamin, L. E. (2005). Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci U S A 102(1): 128-33.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Phung, T. L. and Ayyaswamy, S. (2017). A Novel Mouse Skin Graft Model of Vascular Tumors Driven by Akt1. Bio-protocol 7(13): e2369. DOI: 10.21769/BioProtoc.2369.
Category
Cancer Biology > General technique > Animal models
Cell Biology > Tissue analysis > Tissue imaging
Cancer Biology > General technique > Tumor formation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











