- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Nuclei Isolation from Nematode Ascaris
Published: Vol 7, Iss 9, May 5, 2017 DOI: 10.21769/BioProtoc.2262 Views: 9774
Reviewed by: Gal HaimovichManish ChamoliAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Preparation of Chromatin Fragments From Human Cells for Cryo-EM Analysis
Suguru Hatazawa [...] Hitoshi Kurumizaka
Oct 20, 2025 1965 Views

Optimized Method for High-Quality Isolation of Single-Nuclei From Mosquito Fat Body for RNA Sequencing
Stephanie Serafim de Carvalho [...] Carolina Barillas-Mury
Jan 5, 2026 249 Views

Nuclei Isolation Methods on Frozen Clotted Blood Samples
Melissa Cuevas [...] Nancy Hadley Miller
Jan 20, 2026 306 Views
Abstract
Preparing nuclei is necessary in a variety of experimental paradigms to study nuclear processes. In this protocol, we describe a method for rapid preparation of large number of relatively pure nuclei from Ascaris embryos or tissues that are ready to be used for further experiments such as chromatin isolation and ChIP-seq, nuclear RNA analyses, or preparation of nuclear extracts (Kang et al., 2016; Wang et al., 2016).
Keywords: Nuclei isolationBackground
Nuclei isolation is often the first step in studying the molecular and biochemical aspects of nuclear events. Several methods have been developed to isolate nuclei from different tissues and cell types. However, few nuclei isolation protocols from nematodes other than C. elegans have been described (Ooi et al., 2010; Zanin et al., 2011; Haenni et al., 2012). Embryos of the parasitic nematode Ascaris have been used to prepare a variety of extracts for in vitro cell-free systems (Cohen et al., 2004; Lall et al., 2004). However, these extracts were typically whole cell extracts. Here we describe a method for preparation of nuclei from the nematode Ascaris.
Materials and Reagents
- 15 ml Falcon tubes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 339651 )
- 50 ml Falcon tubes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 339653 )
- 225 ml Falcon bottles (Corning, Falcon®, catalog number: 352075 )
- Ascaris
Ascaris can be collected from slaughterhouses that process several thousand pigs a day and use the small intestines to make sausage casings. Ascaris can be picked up by hands (with gloves) from contents of intestine, which are pushed out to a tank by machines. Usually, it takes two persons 3-5 h to collect ~1,000 worms. Both fresh tissues and embryos can be obtained from these live worms. Female Ascaris can also be ordered from Carolina Biological, Living Zoology Department. These females are shipped dead on ice and the Ascaris zygotes can be obtained from the proximal uteri - Sodium hydroxide (NaOH) (Fisher Scientific, catalog number: S318-1 )
- H2O (purified by Milli-Q® Integral Water Purification System)
- DAPI (Santa Cruz Biotechnology, catalog number: sc-3598 )
- Liquid nitrogen (Airgas, catalog number: NI NF160LT22 )
- Phenylmethanesulfonyl fluoride (PMSF) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 36978 )
- cOmpleteTM protease inhibitor cocktail (Roche Diagnostics, catalog number: 0493116001 )
- Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-3 )
- Potassium chloride (KCl) (Fisher Scientific, catalog number: P217-500 )
- Sodium phosphate dibasic (Na2HPO4) (Fisher Scientific, catalog number: BP350-500 )
- Potassium phosphate monobasic (KH2PO4) (Fisher Scientific, catalog number: P285-500 )
- Hydrochloric acid (HCl)
- 1 M Tris-HCl, pH 8.0 (Thermo Fisher Scientific, AmbionTM, catalog number: AM9856 )
- 1 M Tris-HCl, pH 7.0 (Thermo Fisher Scientific, AmbionTM, catalog number: AM9851 )
- Sodium hypochlorite solution (Fisher Scientific, catalog number: SS290-1 )
- Potassium hydroxide (KOH) (Fisher Scientific, catalog number: P250-1 )
- Magnesium chloride (MgCl2) (Fisher Scientific, catalog number: M33-500 )
- EGTA (Thermo Fisher Scientific, USB, catalog number: 15703 )
- Sucrose (Fisher Scientific, catalog number: BP220-212 )
- DTT (Fisher Scientific, catalog number: BP172-5 )
- Spermine (Sigma-Aldrich, catalog number: S3256 )
- Spermidine (Sigma-Aldrich, catalog number: S2626 )
- Nonidet P-40 (NP-40) (Alfa Aesar, Affymetrix/USB, catalog number: J19628 )
- Triton X-100 (Promega, catalog number: H5142 )
- Glycerol (Fisher Scientific, catalog number: BP229-1 )
- 2-mercaptoethanol (Sigma-Aldrich, catalog number: M6250-10ML )
- EDTA (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 17892 )
- PBS buffer (see Recipes)
- 1 M Tris-HCl, pH 7.8 (see Recipes)
- KOH/Bleach (0.4 N KOH/1.4% sodium hypochlorite) (see Recipes)
- Nuclei extraction buffer A (see Recipes)
- Nuclei extraction buffer B (see Recipes)
- Nuclei extraction buffer C (see Recipes)
- Nuclei storage buffer (see Recipes)
Equipment
- Erlenmeyer flask (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 4112-0125 , 4112-0250 or 4112-0500 )
- 1 L beaker (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 12011000 )
- 4 L plastic beaker (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 12014000 )
- Shaking incubator (example, Lab-Line, model: 4629 )
- Fluorescence microscope (example, Nikon Instruments, model: TS100 )
- Refrigerated centrifuge (example, Thermo Fisher Scientific, model: SorvallTM LegendTM XTR )
- Metal dounce homogenizer (WHEATON, catalog number: 357574 )
- Mortar and pestle (example, Fisher Scientific, catalog numbers: S02591 and S02594 )
Procedure
- Isolation of embryos from Ascaris
- Ascaris embryos dissection
- Note that the anterior end of the female worms is thinner. Looking posterior from the anterior end, identify the genial girdle and cut female worms at or just posterior to the genital girdle (see Figure 1A). Males are typically smaller than females and have a strongly curved posterior (see Figure 1B).
- Gently pull the uteri completely out while holding on the posterior portion of the female until you feel tension. Examine the length of uterus, make cut and collect the proximal ¼ of the uterus into PBS (pH 7.0) in a ~1 L beaker on ice. The length of the proximal region will vary as the overall size of individual female worms can be quite variable (see Figure 1C).

Figure 1. Ascaris embryo dissection. A. Female Ascaris. Light blue arrow indicates the genital girdle; Red arrow is the cutting area. B. Male Ascaris (left) vs. female Ascaris (right), male Ascaris are smaller and have a strong curve in the posterior (black arrow) (Modified from https://en.wikipedia.org/wiki/Ascaris_suum). C. Illustration of female worm cut at the genital girdle with the uteri pulled out. The proximal ¼ (white arrow) of the uterus is collected into PBS. - Dispose of distal 3/4 of uterus.
- Rinse pooled uteri in a beaker three times with ice-cold PBS, pH 7.0 (hand over end to rinse; measure uterus volume in the beaker) (see Figure 2).

Figure 2. Rinsed pooled Ascaris uteri - Treat uteri with 0.5 N NaOH for ~1 h with gentle stirring at room temperature to keep the material suspended.
The ratio of uteri/NaOH is ~50 ml packed uteri/0.6 L of 0.5 N NaOH. Stir to keep all material suspended. - Allow ~45 min for solution to settle by gravity.
- Decant the solution slowly and carefully (as a safety precaution, decanted solution should be autoclaved before discarding). Note that there is often some white floating material present when you decant that you can pour out. The eggs stay down and when you see them nearing the spout stop decanting.
- Repeat the 0.5 N NaOH treatment and decant the supernatant.
- Transfer the egg suspension into 225 ml Falcon bottles. Collect the embryos at 1,250 x g for 10 min at 4 °C.
- Wash the embryos with ice-cold MilliQ water 3-4 times by spinning at 1,250 x g for 10 min at 4 °C.
- Wash with ice-cold PBS (pH 2.0) and collect the embryos at 1,250 x g for 10 min at 4 °C, finally resuspend and store the embryos in 5 volumes of ice-cold PBS (pH 2.0).
Store at 4 °C (The embryos can be stored at 4 °C for at least 3-5 years) (see Figure 3).
Note: Typical embryo yield for proximal uteri is ~20-25 ml/500 worms.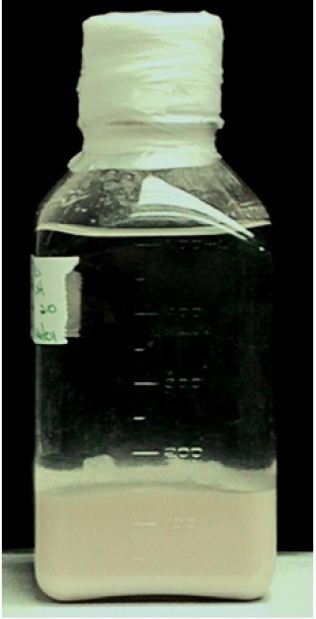
Figure 3. Ascaris embryos. Keep Ascaris embryos in 5 volume of ice-cold PBS, pH 2.0. Store at 4 °C.
- Note that the anterior end of the female worms is thinner. Looking posterior from the anterior end, identify the genial girdle and cut female worms at or just posterior to the genital girdle (see Figure 1A). Males are typically smaller than females and have a strongly curved posterior (see Figure 1B).
- Ascaris embryonation to prepare developmentally staged embryos for nuclei isolation
- Resuspend embryos and remove a volume of suspension appropriate for the experimental purpose (see Table 2 for the amount of nuclei to be used in different types of experiments).
Note: Packed embryo volume will decrease by 60% after removal of the chitinous shell (Coat removal) and washing. - Spin down embryos at 1,250 x g for 5 min at room temperature and discard supernatant.
- Wash embryos with 50 ml of PBS, pH 2.0, spin as above and discard supernatant.
- Resuspend embryos in ~20 volumes PBS, pH 2.0, and place them in sterile, disposable Erlenmeyer flask with cap on loose to facilitate aeration.
Note: Volume of PBS should be no more than 30% of the flask. For example, the maximum volume of PBS should be 75 ml for 250 ml flask. - Incubate embryos in an incubator at 30 °C shaking at 100 rpm for desired time of development (see timing of development Table 1).
Table 1. Ascaris embryonation times at 30 °C with shakingDevelopment time Predominant stage Other notes 0 h 1 cell Prior to pronuclear fusion 24 h 1 cell Pronuclei are fused 46 h 2 cell 2-cell (82%) 62-63 h 2-4 cell 2-cell (16%), 4-cell (49%), and 4-cell + (35%) 96 h 10-26 cell > 90% 114 h 32-64 cell - Examine embryos microscopically to ensure that the embryos have developed to the desired stages.
Note: Embryos through the 8-cell stage of development can be easily staged via light microscopy, whereas later stages typically require DAPI nuclear staining via fluorescence microscopy to define the cell number and developmental stage (Wang et al., 2014).
- Resuspend embryos and remove a volume of suspension appropriate for the experimental purpose (see Table 2 for the amount of nuclei to be used in different types of experiments).
- Chitinous shell (coat) removal of Ascaris embryos
- Pellet embryos (from step A2) in 15 or 50 ml Falcon tubes at 1,250 x g for 5 min at room temperature. Remove supernatant.
- Resuspend embryos in 15-20x pellet volumes or 50 ml of freshly made 0.4 N KOH/1.4% sodium hypochlorite (solution should be pre-warmed to 30 °C).
- Transfer suspension back to original Erlenmeyer flask and incubate for 90 min at 30 °C in incubator shaker with shaking at 100 rpm.
- Pellet embryos in 15 or 50 ml Falcon tubes at 1,250 x g for 5 min at room temperature.
- Wash embryos 5 times with 15-20x pellet volumes of ice-cold PBS, pH 7.0 by gentle resuspension and centrifugation as in step A1d.
- Estimate packed embryo volume from pelleted embryos. The volume of final embryos after decoating is typically about 60% of the starting volume (see Figures 4A and 4B).
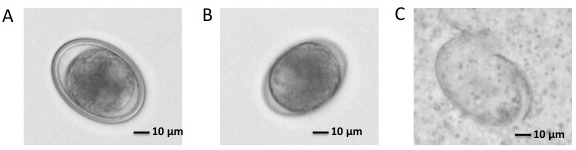
Figure 4. Ascaris embryos. A. One cell stage Ascaris embryo. There is thick chitinous shell (coat) outside the embryo membrane. B. One cell stage Ascaris embryo after coat removal. Chitinous shell is removed and embryo membrane is still there. C. Ascaris embryos after homogenate.
- Pellet embryos (from step A2) in 15 or 50 ml Falcon tubes at 1,250 x g for 5 min at room temperature. Remove supernatant.
- Ascaris embryos dissection
- Preparation of Ascaris tissue for nuclei isolation
- Ascaris dissection
Ascaris can be collected from a pig slaughterhouse and placed in 30 °C PBS (pH 7.0) while being transported to the lab. It is important to keep the worms at 30 °C to maintain viability.
Note: Ascaris can be kept alive in 30 °C PBS, pH 7.0 for at least 2-3 days. However, it is better to dissect the worms on the same day to get fresh tissues.- Rinse worms with warm PBS, pH 7.0.
- Male Ascaris can be dissected to obtain spermatids, intestine, testis, and the remaining tissue (the carcass, which includes muscle, hypodermis, pharynx, and neurons). Female Ascaris can be dissected to obtain ovary/oviduct, oocytes, uterus, intestine, and carcass. Tissues should be frozen in liquid nitrogen (without PBS), and stored at -80 °C. Note that the reproductive systems can be up to a meter long and different regions can be identified and subdivided for analysis.
- Rinse worms with warm PBS, pH 7.0.
- Ascaris dissection
- Nuclei isolation
- Nuclei isolation from Ascaris embryos.
- Suspend ~900 μl of decoated embryos in 10 ml of nuclei extraction buffer A and centrifuge at 1,200 x g for 5 min at 4 °C. Note that ~10 million embryos can be obtained from ~1 ml packed decoated embryos (~20 mature female Ascaris). The number of nuclei will depend on the developmental stage of the embryos. (See Table 2 for the amount of nuclei necessary for different types of experiments.)
Table 2. Protein, RNA, and DNA content as a function of Ascaris nuclei and amounts needed for ChIP experimentsExperiments Nuclear protein (10 μg) Nuclear RNA (10 μg) DNA (10 ng) ChIP/ChIP-seq Nuclei ~105 ~105 ~104 ~106 - Remove supernatant. Resuspend the 900 μl cell pellet in 10 ml of ice-cold nuclei extraction buffer B with 0.5 mM PMSF and cOmpleteTM protease inhibitor cocktail. Transfer the suspension into a 15 ml WHEATON metal dounce homogenizer kept on ice.
- Homogenize with 10 passes.
Note: Examine embryos under a microscope to make sure over 80% are broken. If not, homogenize further (see Figure 4C). - Transfer the suspension to 15 ml Falcon tubes and spin at 750 x g for 10 min at 4 °C in Sorvall Legend XT with swinging bucket rotor. Remove supernatant.
Notes:- Comparison of the number of nuclei in the supernatant to pellet (equivalent volumes) should show very high nuclei enrichment in pellets (some nuclei remain in supernatant).
- If you want to collect a cytoplasmic extract, retain the supernatant. For cytoplasmic extracts, we typically clarify the supernatant at 21,000 x g at 4 °C for 15 min, transfer aliquouts to pre-cooled microfuge tubes, freeze the tubes in liquid nitrogen, and store at -80 °C.
- Comparison of the number of nuclei in the supernatant to pellet (equivalent volumes) should show very high nuclei enrichment in pellets (some nuclei remain in supernatant).
- Resuspend nuclear pellets in 10 ml of ice-cold nuclei extraction buffer A, centrifuge at 750 x g for 10 min at 4 °C in Sorvall Legend XT with swinging bucket rotor. Repeat twice and after the 3rd centrifugation step remove as much supernatant as possible from the nuclear pellet.
- Resuspend pellets in 10 ml of ice cold nuclei extraction buffer C, centrifuge at 2,000 x g for 15 min at 4 °C in Sorvall Legend XT in swinging bucket rotor. Remove supernatant and examine supernatant for nuclei. The majority of nuclei should be in pellet.
- The isolated nuclei can be used immediately or frozen in storage buffer in liquid nitrogen and stored at -80 °C for future experiments.
Note: See isolation of Ascaris embryos, embryonation, and preparation of embryos for nuclei isolation in Procedure C.
- Suspend ~900 μl of decoated embryos in 10 ml of nuclei extraction buffer A and centrifuge at 1,200 x g for 5 min at 4 °C. Note that ~10 million embryos can be obtained from ~1 ml packed decoated embryos (~20 mature female Ascaris). The number of nuclei will depend on the developmental stage of the embryos. (See Table 2 for the amount of nuclei necessary for different types of experiments.)
- Nuclei isolation from Ascaris tissue
- Grind Ascaris tissue from 3-5 worms (~105-106 nuclei, depends on differences in the tissues) to a fine powder in liquid nitrogen using mortar and pestle. (See Table 2 for amount of nuclei should be used for different purpose of experiments.)
- Wash Ascaris tissue powder in 10 ml of ice-cold PBS and centrifuge at 1,200 x g for 5 min at 4 °C.
- Repeat step C2b twice.
- Isolate nuclei as described above in Procedure A.
Note: The volume of buffer at each step should be optimized based on volume of tissue powder, use the same amount of buffer as used for the decoated embryos.
- Grind Ascaris tissue from 3-5 worms (~105-106 nuclei, depends on differences in the tissues) to a fine powder in liquid nitrogen using mortar and pestle. (See Table 2 for amount of nuclei should be used for different purpose of experiments.)
- Nuclei isolation from Ascaris embryos.
Notes
- Dissection of 1,500 worms takes ~4-5 h for 2 people. For efficient dissection and preventing bacterial growth, isolate embryos immediately upon worms and keep them cold.
- Ascaris is known to cause infection in human; hence proper precautionary measure should be followed to protect yourself and other lab members from infection. It has been reported that the pig Ascaris, Ascaris suum, can infect humans leading to pulmonary infection. Isolation of eggs from female Ascaris is not a direct infection threat. The infective stages of Ascaris are the L2/L3 stages that develop inside eggs. Ascaris eggs isolated from the uteri of females require 3-4 weeks development at 30 °C with appropriate aeration to become infective. Thus, if the embryos are not embryonated to infective larvae, they do not impose an infection risk. Although unlikely and very rare, it remains possible that some egg contamination could occur during their isolation from the uteri of females. Therefore, safety protocols to protect laboratory workers including personal protection devices, dedicated work places, sterilization of material coming in contact with Ascaris, and treatment of benches and material to remove Ascaris eggs to prevent their development to infective stages as followed. Finally, exposure to adult worms may lead to anaphylactic shock. Lab personnel should be informed of all the risks of working with Ascaris including symptoms of anaphylactic shock and its treatment. In addition, as a precaution people should work in pairs with live adult worms to ensure that another person is present in the event that someone develops anaphylactic shock.
- Depending on the tissues and size of the nuclei this protocol can be modified and optimized to work with other nematodes.
- The large size of the Ascaris allows different parts of the worm tissues to be isolated and used for nuclei extraction. However, for most other small nematodes, the whole worm is used for nuclei isolation.
Recipes
- PBS buffer
8 g NaCl
0.2 g KCl
1.44 g Na2HPO4
0.24 g KH2PO4
Add ddH2O up to 1 L
Adjust the pH to 7.0 or 2.0 with HCl - 1 M Tris-HCl, pH 7.8
15 ml 1 M Tris-HCl, pH 7.0
16 ml 1 M Tris-HCl, pH 8.0 - KOH/Bleach (0.4 N KOH/1.4% sodium hypochlorite)
35.2 ml of sodium hypochlorite solution
2.24 g KOH
Add ddH2O up to 100 ml - Nuclei extraction buffer A
1.5 mM MgCl2
1 mM EGTA
0.5 M sucrose
10 mM KCl
20 mM Tris-HCl, pH 7.8
1 mM DTT
1 mM spermine
1 mM spermidine
Add ddH2O to desired final volume - Nuclei extraction buffer B
Add the list to nuclei extraction buffer A
0.15% NP-40
0.1% Triton X-100
Add ddH2O to desired final volume
Add 0.5 mM PMSF and 1x cOmpleteTM protease inhibitor cocktail immediately prior use - Nuclei extraction buffer C
1.5 mM MgCl2
1 mM EGTA
1 M sucrose
10 mM KCl
20 mM Tris-HCl, pH 7.8
1 mM DTT
1 mM spermine
1 mM spermidine
Add dd H2O to desired final volume - Nuclei storage buffer
40% glycerol
50mM Tris-HCl
5mM MgCl2
0.05% 2-mercaptoethanol
1 mM DTT
1 mM EDTA
Add ddH2O to desired final volume
Acknowledgments
This work was supported in part by NIH grants to R.E.D. (AI0149558 and AI114054).
References
- Cohen, L. S., Mikhli, C., Friedman, C., Jankowska-Anyszka, M., Stepinski, J., Darzynkiewicz, E. and Davis, R. E. (2004). Nematode m7GpppG and m32,2,7GpppG decapping: activities in Ascaris embryos and characterization of C. elegans scavenger DcpS. RNA 10(10): 1609-1624.
- Haenni, S., Ji, Z., Hoque, M., Rust, N., Sharpe, H., Eberhard, R., Browne, C., Hengartner, M. O., Mellor, J., Tian, B. and Furger, A. (2012). Analysis of C. elegans intestinal gene expression and polyadenylation by fluorescence-activated nuclei sorting and 3'-end-seq. Nucleic Acids Res 40(13): 6304-6318.
- Kang, Y., Wang, J., Neff, A., Kratzer, S., Kimura, H. and Davis, R. E. (2016). Differential chromosomal localization of centromeric histone CENP-A contributes to nematode programmed DNA elimination. Cell Rep 16(9): 2308-2316.
- Lall, S., Friedman, C. C., Jankowska-Anyszka, M., Stepinski, J., Darzynkiewicz, E. and Davis, R. E. (2004). Contribution of trans-splicing, 5' -leader length, cap-poly(A) synergism, and initiation factors to nematode translation in an Ascaris suum embryo cell-free system. J Biol Chem 279(44): 45573-45585.
- Ooi, S. L., Henikoff, J. G. and Henikoff, S. (2010). A native chromatin purification system for epigenomic profiling in Caenorhabditis elegans. Nucleic Acids Res 38(4): e26.
- Wang, J., Garrey, J. and Davis, R. E. (2014). Transcription in pronuclei and one- to four-cell embryos drives early development in a nematode. Curr Biol 24(2): 124-133.
- Zanin, E., Dumont, J., Gassmann, R., Cheeseman, I., Maddox, P., Bahmanyar, S., Carvalho, A., Niessen, S., Yates, J. R., 3rd, Oegema, K. and Desai, A. (2011). Affinity purification of protein complexes in C. elegans. Methods Cell Biol 106: 289-322.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kang, Y., Wang, J. and Davis, R. E. (2017). Nuclei Isolation from Nematode Ascaris. Bio-protocol 7(9): e2262. DOI: 10.21769/BioProtoc.2262.
Category
Cell Biology > Organelle isolation > Nuclei
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









