- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse Model of Reversible Intestinal Inflammation
Published: Vol 7, Iss 6, Mar 20, 2017 DOI: 10.21769/BioProtoc.2173 Views: 12774
Reviewed by: Ivan ZanoniAlesssandro ArduiniMareta Ruseva

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vivo Electroporation of Skeletal Muscle Fibers in Mice
Steven J. Foltz [...] Hyojung J. Choo
Jul 5, 2023 1823 Views

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3758 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2626 Views
Abstract
Current therapies to treat inflammatory bowel disease by dampening excessive inflammatory immune responses have had limited success (Reinisch et al., 2011; Rutgeerts et al., 2005; Sandborn et al., 2012). To develop new therapeutic interventions, there is a need for better understanding of the mechanisms that are operative during mucosal healing (Pineton de Chambrun et al., 2010). To this end, a reversible model of colitis was developed in which colitis induced by adoptive transfer of naïve CD4+ CD45RBhi T cells in lymphopenic mice can be reversed through depletion of colitogenic CD4+ T cells (Brasseit et al., 2016).
Keywords: ColitisBackground
Our understanding of the pathogenesis of inflammatory bowel disease (IBD), which is a chronic inflammatory disorder of the intestine, has been greatly improved with the development of animal models aiming to recapitulate human disease (Khanna et al., 2014). Despite the identification of a wide array of immunological targets, current therapies have had limited success in treating IBD and limited knowledge is available about the mechanisms that are induced in the establishment of long-term remission and the associated mucosal healing (D’Haens et al., 2014). A major limitation, so far, has been the lack of animal models in which remission can be reproducibly induced in animals with established disease. In models of infection induced intestinal inflammation, pro-inflammatory and anti-inflammatory mechanisms can be operative simultaneously, implying that dissecting the role of different immune pathways during resolution of inflammation may be a challenge (Endt et al., 2010; Sonnenberg et al., 2011). In dextran sodium sulfate (DSS) induced colitis, DSS can be easily administered in the drinking water to either induce acute or chronic intestinal inflammation and this is followed by treatment with normal drinking water to study resolution of colitis. However, the kinetics and severity of disease is highly dependent on numerous factors including differences in the dose of DSS used and critically on the amount of DSS consumed which is virtually impossible to normalize between different animals of the same cage (Chassaing et al., 2014; Perše and Cerar, 2012). Using the T cell transfer mediated colitis model, it was elegantly shown that intestinal inflammation can be reversed by the adoptive transfer of CD45RBlo regulatory T cells (Treg) in colitic animals, resulting in remission induction within 10-14 weeks post Treg transfer (Mottet et al., 2003). The kinetics of remission induction however varies depending on the expansion of transferred Treg and it can be difficult to synchronize the onset of remission between animals of same experimental group. To overcome the unpredictable timing and extent of remission induction, we developed a new mouse model of reversible intestinal inflammation in which intestinal inflammation (induced by the adoptive transfer of naïve CD45RBhi T cells in lymphopenic animals) can be reversed by depletion of colitogenic CD4+ T cells in mice with established disease, resulting in reproducible induction of remission from colitis (Brasseit et al., 2016).
Materials and Reagents
- 50 ml tube (SARSTEDT, catalog number: 62.547.254 )
- 5 ml Falcon polystyrene (PS) round bottom tube (Corning, Falcon®, catalog number: 352058 )
- 100 µm nylon cell strainer (Corning, catalog number: 431752 )
- Glass slide Superfrost (Biosystems, catalog number: 85-0551-00 )
- 6-10 week old congenic CD90.1 or CD45.1 mice as donors (in-house bred mice on C57BL/6 background, initially purchased from THE JACKSON LABORATORY, USA)
Notes: - Congenic mice CD90.1 are used as donors to easily distinguish between donor (CD90.1) and recipient cells (CD90.2) but C57BL/6 mice can also be used as donors.
- It is possible to use donor mice older than 10 weeks old but the frequency of naïve CD4+ CD45RBhi T cells is expected to decrease with age.
- 10-16 week old Helicobacter positive C57BL/6 Rag2-/- or Rag1-/- mice (initially purchased from THE JACKSON LABORATORY, USA) housed under specific pathogen-free (SPF) conditions as recipients with a minimum of 20 g body weight
Notes: - Helicobacter negative SPF Rag2-/- (or Rag1-/-) mice may also be used as recipients. However, the kinetics of colitis induction are delayed and the variation in disease activity may greatly vary at a given time post CD4 T cell-mediated colitis induction in those Helicobacter negative mice.
- Positivity for the presence of Helicobacter can be assessed by PCR using genomic DNA extracted from fecal pellets as previously described by Brasseit et al., 2016.
- We have not observed any differences in the kinetics of colitis induction between male and female recipients.
- 0.5 M EDTA (Sigma-Aldrich, catalog number: 27285 ), in sodium form
- EasySepTM Mouse Streptavidin RapidSpheresTM Isolation Kit (STEMCELL Technologies, catalog number: 19860 )
- InVivoMAb anti-mouse CD4 (Clone GK1.5) (BioxCell, catalog number: BE0003-1 )
- Anti-mouse antibodies
Antibodies Company Catalog number Clone Dilution B220 Biotin BioLegend 103203 RA3-6B2 1:800 CDαBiotin BioLegend 100703 53-6.7 1:200 CD45RB FITC Affymetrix 11-0455-85 C363-16A 1:800 CD25 PE BioLegend 102008 PC61 1:800 CD4 APC-Cy7 BioLegend 100526 RM4-5 1:800 - Horse serum (HS) (Sigma-Aldrich, catalog number: H1270 )
- Trypan blue solution (Sigma-Aldrich, catalog number: T8154 )
- 4% buffered formalin (EMD Millipore, catalog number: 100496 )
- Ethanol (EMD Millipore, catalog number: 100983 )
- Xylene (VWR, catalog number: 28975 )
- Paraffin (Engelbrecht, catalog number: 17932 )
- Mayer’s hemalum (Hematoxylin) solution (EMD Millipore, catalog number: 109249 )
- Xylene based mounting medium Eukitt® (Sigma-Aldrich, catalog number: 03989 )
- Sodium chloride (NaCl) (EMD Millipore, catalog number: 106406 )
- di-Sodium hydrogen phosphate dehydrate (Na2HPO4·2H2O) (EMD Millipore, catalog number: 106580 )
- di-Potassium hydrogen phosphate trihydrate (K2HPO4·3H2O) (EMD Millipore, catalog number: 105099 )
- Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: 31107 )
- Potassium hydrogen carbonate (KHCO3) (EMD Millipore, catalog number: 104854 )
- Eosin-Phloxine solution (VWR, catalog numbers: 341973R and 10047229 , respectively)
- Glacial acetic acid (EMD Millipore, catalog number: 100063 )
- Hydrochloric acid (EMD Millipore, catalog number: 100317 )
- Phosphate-buffered saline (PBS) (see Recipes)
- ACK red blood lysis buffer (see Recipes)
- Eosin-Phloxine solution (see Recipes)
- 0.5% HCl-ethanol (see Recipes)
Equipment
- Centrifuge
- Neubauer chamber (Hemocytometer)
- EasySepTM magnet (STEMCELL Technologies, catalog number: 18000 )
- BD FACS ARIA III (BD, model: BD FACS ARIA III )
- Tissue-Tek VIP 6 tissue processor (SAKURA, model: Tissue-Tek VIP 6 Vaccum Infiltration Processor )
- Tissue-Tek®uni-cassette® (SAKURA, catalog number: 4172 )
- Heating block
- Dissection kit (forceps and scissors)
- Cold plate (-10 °C)
- Microtome (Leica Biosystems, Wetzlar, Germany)
- Water bath
- Pipette
- CO2 chamber
Note: CO2 is used as an approved and recommended euthanasia method in accordance with the Swiss Federal and Cantonal animal experimental regulations.
Software
- Graphpad Prism 6 (La Jolla, CA; https://www.graphpad.com/scientific-software/prism/)
Procedure
- Selection of naïve CD4+ T cells
- On day 0, isolate spleens from donor CD90.1 mice. Use 1 spleen per 2 recipient Rag1-/- mice.
- Disaggregate spleens on a 100 µm nylon filter and rinse the filter with PBS/2% horse serum (HS) to obtain a single cell suspension.
- Spin down cell suspension at 370 x g for 5 min.
- Lyse the red blood cells by resuspending the cell pellet in 3 ml of ACK lysis buffer for 3 min.
- Wash cells with PBS/2%HS and resuspend the cell pellet in 1 ml of PBS/2% HS containing 5 mM EDTA.
Note: The initial volume for cell resuspension is not critically important since the cells have to be counted and adjusted to 1 x 108 cells/ml, as indicated in the following step. - Count the cell numbers using a hemocytometer and adjust the cell concentration to 1 x 108 cells/ml.
- Add 50 µl/ml of the provided rat serum (from EasySepTM Mouse Streptavidin RapidSpheresTM Isolation Kit) and incubate with biotinylated antibodies (anti-mouse B220 and CD8α). Mix the cells carefully by pipetting.
- Leave the tube containing the cell suspension on ice for 15 min.
Note: Cells have to be mixed carefully by pipetting following addition of anti-mouse antibodies. The tube containing the cell resuspension can be left on ice without further mixing/inverting/shaking. - Vortex the streptavidin RapidSpheresTM for 30 sec and add 85 µl/ml of RapidSpheresTM to the cell suspension.
Note: No initial wash step of the RapidSpheresTM is required but vortexing the RapidSpheresTM is critical since they tend to settle at the bottom of the tube. - Mix carefully by pipetting up and down and incubate at room temperature for 2.5 min.
- Bring the cell suspension to a total volume of 2.5 ml using PBS/2% HS containing 5 mM EDTA and transfer to a 5 ml PS tube.
- Place the 5 ml PS tube containing the cell suspension into the EasySepTM magnet for 2.5 min at room temperature.
- In one continuous motion, invert the magnet and carefully pour off the cell fraction (streptavidin RapidSpheresTM unbound fraction) into a new 5 ml tube, leaving the magnet and tube in an inverted position for 2-3 sec to ensure optimal transfer of the cell suspension.
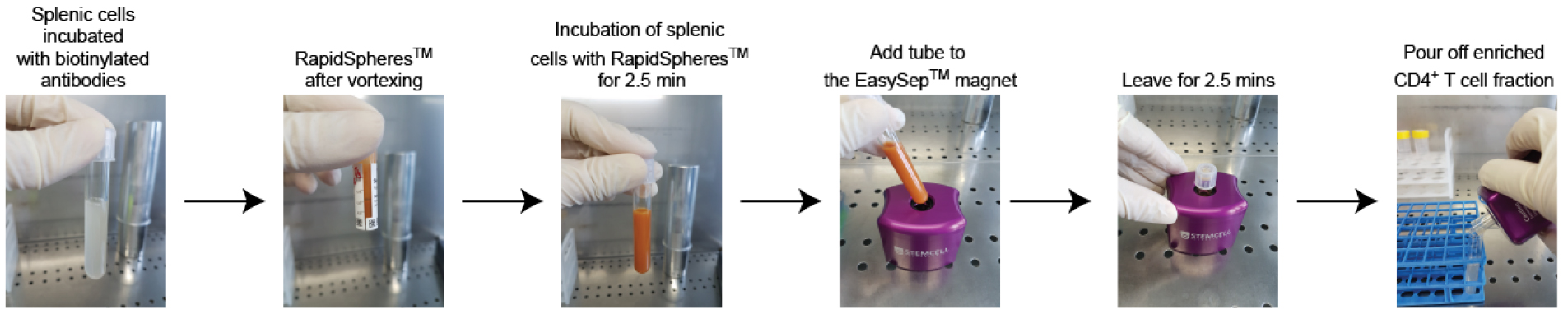
Figure 1. T cell enrichment method using splenocytes of donor mice - Wash cells in PBS/2% HS at 370 x g for 5 min.
- Stain cells in 1 ml of anti-mouse CD45RB FITC, CD25 PE and CD4 APC-Cy7. Mix the cells carefully by pipetting. Leave the tube containing the cell suspension on ice for 20 min without further mixing/inverting/shaking.
- Wash cells (enriched for CD4+ T cells) with PBS/2% HS prior to cell sorting for CD4+CD45RBhi T cells on a BD FACSARIA III cell sorter.
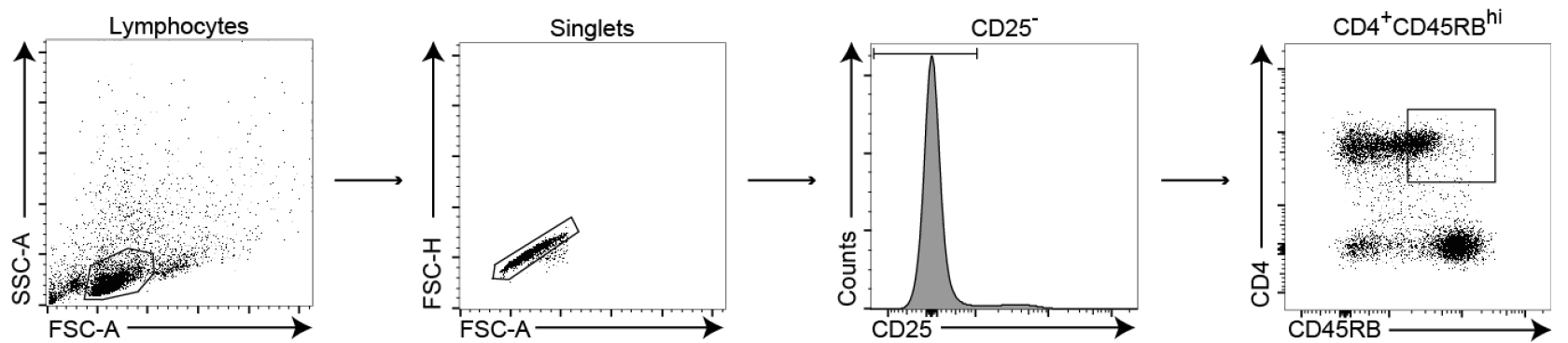
Figure 2. Gating strategy for the selection of naïve CD45RBhi T cells from spleens of donor C57BL/6 congenic mice. Lymphocytes are first selected based on their forward and side scatter properties, followed by the exclusion of doublet cells. Regulatory and activated T cells are excluded by selecting CD25 negative lymphocytes from the singlets gate and finally, naïve CD4+ T cells are selected based on their high expression of CD4 and CD45RB. - Collect sorted cells in 1 ml of HS (100% HS).
- Wash sorted CD4+CD45RBhi T cells in PBS for 5 min at 370 x g and resuspend in PBS at a concentration of 1 x 106 cells/ml.
Note: Keep the cells on ice at all times. If sorted naïve CD4+ T cells cannot be injected right away, keep the cells in HS on ice. Only wash and resuspend the cells with PBS prior to injection. - To induce colitis, inject C57BL/6 Rag2-/- mice with 2 x 105 CD4+CD45RBhi T cells in a volume of 200 µl intraperitoneally (i.p).
- Once the animals are colitic (see Procedure B), inject the animals three times with 250-500 µg of anti-CD4 depleting antibody with an interval of 72 h between each injection to induce remission.
Note: Remission induction was initially performed using 500 µg of αCD4 depleting antibody, as described by Brasseit et al., 2016. In our experiments, we can still achieve the same level of CD4+ T cell depletion and similar kinetics of remission induction using 3 treatments of 250 µg αCD4 depleting antibody. - Monitor the animals daily for weight change and normal activity, with relapse of colonic inflammation expected around 22-25 days (in Helicobacter positive recipients) after the first treatment with anti-CD4 depleting antibody.
Note: To assess the development of colitis, monitor the mice daily since the kinetics of colitis induction is strongly dependent on the composition of the microbiota implying that disease progression may vary from animals of one SPF mouse house facility to the other. In our experience, clinical and histopathological signs of colitis are seen around day 9-10 post T cell transfer in Helicobacter positive mice and around day 30 post T cell transfer in Helicobacter negative mice. In Helicobacter positive recipient mice, clear weight loss (approximately 10%) and appearance of colitogenic CD4+ T cells in the blood occurs very rapidly from one day to the other. Moreover, we generally observe that the weight loss correlates strongly with the extent of intestinal inflammation in Helicobacter positive but not in Helicobacter negative recipients.
- On day 0, isolate spleens from donor CD90.1 mice. Use 1 spleen per 2 recipient Rag1-/- mice.
- Clinical and histopathological scoring
- Mice with active intestinal inflammation (clinical score ≥ 8) and with detectable CD4+ T cells in the blood are considered colitic. For the measurement of CD4+ T cells in the blood, collect 2-3 drops of blood by the tail vein in 1 ml of PBS/2% HS/5 mM EDTA. Spin down the cells by centrifugation at 370 x g for 5 min. Resuspend the cells in 100 µl of PBS/2% HS containing anti-mouse CD4 antibody (Refer to Materials and Reagents section for antibody dilution) by pipetting. Following incubation on ice for 15 min, wash the cells in PBS/2% HS and analyse on a flow cytometer.
- For clinical disease scoring, monitor the following parameters daily:
- Weight loss
- Stool consistency
- Appearance of blood in the stool
- Behavior
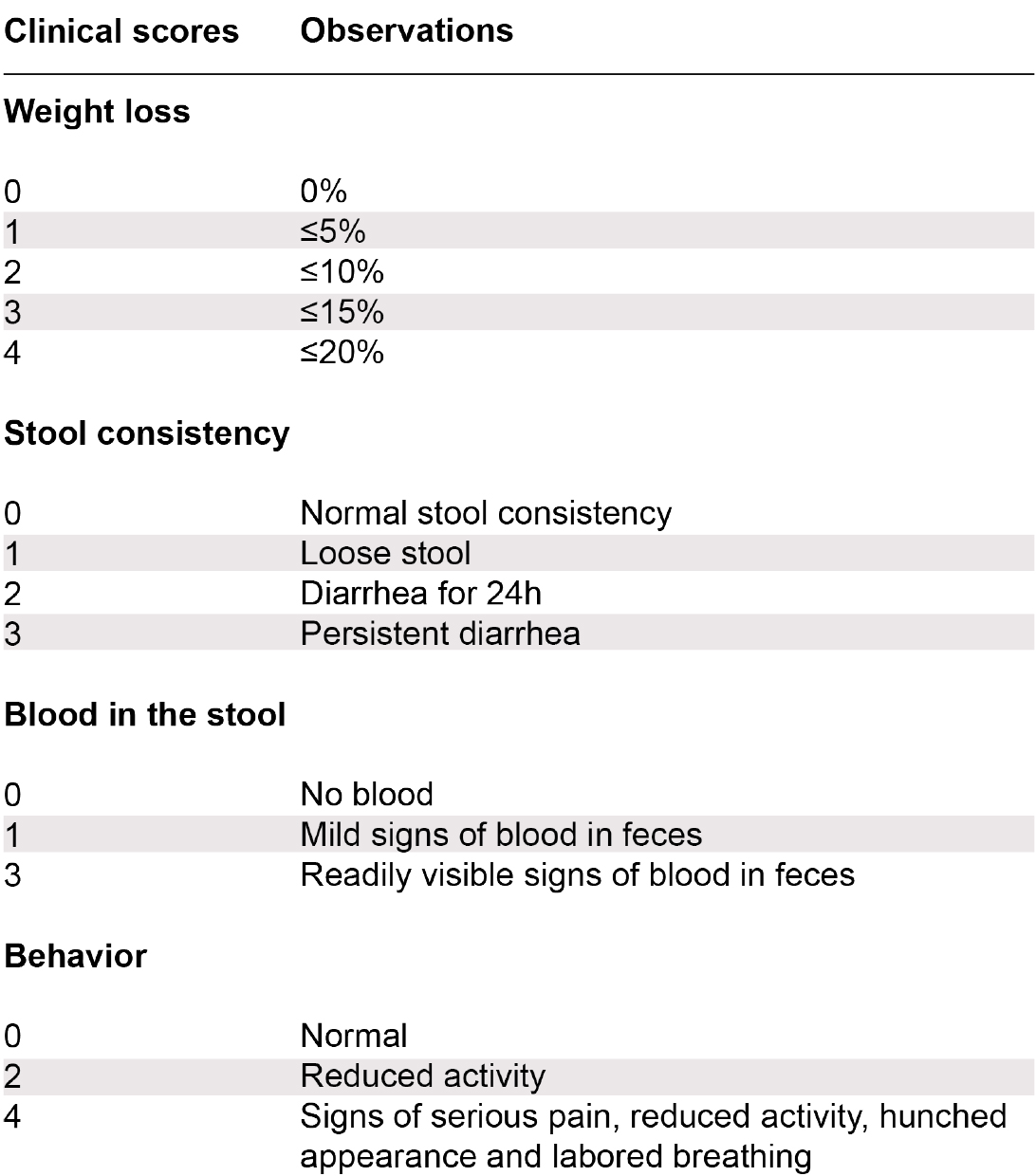
Figure 3. Scoring system used to assess colitic animals following transfer of CD4+ CD45RBhi T cells in lymphopenic recipients. Mice with a clinical score of ≥ 8 were considered colitic (multiple combinations leading to a clinical score of 8 are possible). - For histological disease scoring, collect colon (middle) sections for hematoxylin and eosin (H&E) staining.

Figure 4. Collection of colon tissue section for histology - In a blinded manner, measure the following parameters (this should be done by an experienced pathologist). Refer to the publication by Brasseit et al., 2016 in Mucosal Immunology for more details:
- Cellular infiltrates in the lamina propria (Score from 0 to 3)
- Loss of goblet cells (Score from 0 to 3)
- Presence of crypt abscess (Score from 0 to 3)
- Epithelial erosion (Score from 0 to 1)
- Hyperemia (Score from 0 to 2)
- Thickness of the colonic mucosa (Score from 0 to 3)
- Mice with active intestinal inflammation (clinical score ≥ 8) and with detectable CD4+ T cells in the blood are considered colitic. For the measurement of CD4+ T cells in the blood, collect 2-3 drops of blood by the tail vein in 1 ml of PBS/2% HS/5 mM EDTA. Spin down the cells by centrifugation at 370 x g for 5 min. Resuspend the cells in 100 µl of PBS/2% HS containing anti-mouse CD4 antibody (Refer to Materials and Reagents section for antibody dilution) by pipetting. Following incubation on ice for 15 min, wash the cells in PBS/2% HS and analyse on a flow cytometer.
- Paraffin embedding and H&E staining
Note: The paraffin embedding and H&E staining are routinely carried out in our histology core lab facility using a fully automated procedure on a Tissue-Tex VIP 6 but the basic steps are described below.- Collect 1 cm colonic tissue (middle section) in a uni-cassette® and fix in 4% buffered formalin overnight at room temperature.
- Process the tissue as follows (using an automated tissue processor):
- 4% buffered formalin for 1.25 h at 35 °C.
- Distilled water for 15 min at room temperature.
- 70% ethanol for 1.5 h at 35 °C.
- 80% ethanol for 1 h at 35 °C.
- 95% ethanol for 1.5 h at 35 °C.
- 100% ethanol for 1 h at 35 °C (three changes of 1 h each).
- Xylene for 1 h at 35 °C (two changes of 1 h each).
- Paraffin for 45 min at 60 °C (four changes of 45 min each).
- 4% buffered formalin for 1.25 h at 35 °C.
- To embed in a paraffin block. Choose an appropriate mold that corresponds to the size of the cassette.
- Open the cassette and discard the lid.
- Add some liquid paraffin into the mold, which is placed on a heating block at 60 °C to prevent the paraffin from solidifying.
- Transfer the tissue into the mold using warm forceps such that the forceps can be used to transfer the tissue from the cassette to the mold without solidifying the paraffin.
- Place the mold on a cold plate (-10 °C) to start solidifying the paraffin and immediately press the tissue down with forceps.
- Place the tissue cassette on top of the mold and press firmly. Ensure that there is sufficient paraffin to cover the plastic cassette.
- Wait for the paraffin to solidify such that the paraffin block can easily come out of the mold.
- Cut 5 µm sections using a microtome. Briefly, insert the blade in the holder and adjust the angle of the blade to 5°. Place the paraffin block in the object clamp and adjust to the desired orientation. Cut a few sections (10 µm) to expose the tissue surface. Once the tissue is exposed and the tissue cuts are smooth, adjust the section thickness to 5 µm. Carefully pick up the 5 µm tissue sections with forceps and float them in a warm water bath at 37 °C. Collect the sections on a clean glass slide and dry on a heating plate at 40 °C for 20 min.
- To stain with hematoxylin and eosin, first deparaffinize the slides with xylene for 5 min.
- Rinse twice with xylene and rehydrate the slides by rinsing sequentially in:
- Absolute ethanol (two changes);
- 95% ethanol
- 70% ethanol
- Distilled water
- Absolute ethanol (two changes);
- Stain with Mayer’s hemalum solution for 7 min and rinse with tap water.
- Bleach with 0.5% HCl-ethanol for a few seconds (depending on the desired staining depth of the nucleus) and wash slide immediately with tap water followed by a bluing step in tap water for 10 min.
- Rinse with 70% ethanol.
- Counter stain using Eosin-Phloxine solution for 2-3 min. Rinse twice with 70% ethanol.
- Dehydrate the slides by rinsing sequentially in:
- 70% ethanol
- 95% ethanol
- Absolute ethanol (two changes in total)
- Xylene (two changes in total)
- 70% ethanol
- Mount with Eukitt®
- Collect 1 cm colonic tissue (middle section) in a uni-cassette® and fix in 4% buffered formalin overnight at room temperature.
Data analysis
In the design of the experimental set-up, it is recommended to include 4-5 mice/group and perform 2-3 independent experiments. For data analysis, compare recipient mice that received naïve CD45RBhi T cells (experimental group) to those that received CD45RBlo T cells (control group, no colitis). To assess the statistical significance, use an unpaired t-test on GraphPad Prism 6 (La Jolla, CA).
Notes
- To ensure the best cell yield and viability, always work on ice unless indicated otherwise.
- To get rid of the supernatant following centrifugation, aspirate the supernatant and discard rather than pouring it off since cells may not adhere strongly to the bottom of the 5 ml PS tube.
- For cell resuspension, this should be performed gently using a pipette and not by vortexing.
- Prior to cell injection, mix the cells by inverting the tube up and down to ensure that all mice are injected with the same number of cells.
- For intraperitoneal injection, ensure that the needle is inserted properly in the peritoneal cavity prior to injecting the cells.
- If Rag-/- recipients are imported from another mouse house facility, it is highly advisable to house these mice for at least two weeks prior to naïve CD4+ T cell transfer to allow stabilization of the microbiota.
Recipes
- Phosphate-buffered saline (PBS)
137 mM NaCl
1.69 mM Na2HPO4·2H2O
8.63 mM K2HPO4·3H2O - ACK red blood lysis buffer
150 mM NH4Cl
10 mM KHCO3
0.1 mM EDTA - Eosin-Phloxine solution
100 ml of 1% eosin stock solution
10 ml of 1% phloxine stock solution
780 ml of 95% ethanol
4 ml glacial acetic acid - 0.5% HCl-ethanol
20 ml of 25% hydrochloric acid
980 ml of 70% ethanol
Acknowledgments
This work was supported by the Swiss National Science Foundation Sinergia grant to Andrew Macpherson, Christoph Mueller, Wolf-Dieter Hardt and Daniela Finke as well as by the SNSF grant No. 31-138392 to C.M. The described protocol was first outlined in Brasseit et al., 2016.
References
- Brasseit, J., Althaus-Steiner, E., Faderl, M., Dickgreber, N., Saurer, L., Genitsch, V., Dolowschiak, T., Li, H., Finke, D., Hardt, W. D., McCoy, K. D., Macpherson, A. J., Corazza, N., Noti, M. and Mueller, C. (2016). CD4 T cells are required for both development and maintenance of disease in a new mouse model of reversible colitis. Mucosal Immunol 9(3): 689-701.
- Chassaing, B., Aitken, J. D., Malleshappa, M., and Vijay-Kumar, M. (2014). Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104: Unit 15 25
- D’Haens, G. R ., Sartor, R. B ., Silverberg, M. S., Petersson, J. and Rutgeerts, P. (2014). Future directions in inflammatory bowel disease management. J Crohns Colitis 8(8):726-734
- Endt, K., Stecher, B., Chaffron, S., Slack, E., Tchitchek, N., Benecke, a., Van Maele, L., Sirard, J. C., Mueller, A. J., Heinkenwalder, M., Macpherson, A. J., Strugnell, R., Von Mering, C. and Hardt, W. D. (2010). The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog 6(8): e1001097.
- Khanna, P. V., Shih, D. Q., Haritunians, T., McGovern, D. P. and Targan, S. (2014). Use of animal models in elucidating disease pathogenesis in IBD. Semin Immunopathol 36(5): 541-551.
- Mottet, C., Uhlig, H. H. and Powrie, F. (2003). Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 170(8): 3939-3943.
- Perše, M. and Cerar, A (2012). Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol 2012: 718617.
- Pineton de Chambrun, G., Peyrin-Biroulet, L., Lemann, M. and Colombel, J. F. (2010). Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol 7(1): 15-29.
- Reinisch, W., Sandborn, W. J., Hommes, D. W., D’Haens, G., Hanauer, S., Schreiber, S., Panaccione, R., Fedorak, R. N., Tighe, M. B., Huang, B., Kampman, W., Lazar, A. and Thakkar, R. (2011). Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 60(6): 780-787.
- Rutgeerts, P., Sandborn, W. J., Feagan, B. G., Reinisch, W., Olson, A., Johanns, J., Travers, S., Rachmilewitz, D., Hanauer, S. B., Lichtenstein, G. R., de Villiers, W. J., Present, D., Sands, B. E. and Colombel, J. F. (2005). Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353(23): 2462-2476.
- Sandborn, W. J., van Assche, G., Reinisch, W., Colombel, J. F., D'Haens, G., Wolf, D. C., Kron, M., Tighe, M. B., Lazar, A. and Thakkar, R. B. (2012). Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 142(2): 257-265 e251-253.
- Sonnenberg, G.F., Monticelli, L.A., Elloso, M.M., Fouser, L.A., and Artis, D. (2011). CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34(1): 122-134.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kwong Chung, C. K., Brasseit, J., Althaus-Steiner, E., Rihs, S. and Mueller, C. (2017). Mouse Model of Reversible Intestinal Inflammation. Bio-protocol 7(6): e2173. DOI: 10.21769/BioProtoc.2173.
Category
Immunology > Mucosal immunology > Digestive tract
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









