- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Olfactory Avoidance Test (Mouse)
Published: Vol 7, Iss 5, Mar 5, 2017 DOI: 10.21769/BioProtoc.2153 Views: 9566
Reviewed by: Soyun KimAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1294 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1470 Views
Abstract
In mice, olfaction plays a pivotal role for the various behaviors, such as feeding, mating, nursing and avoidance. Behavioral tests that analyze abilities of odor detection and recognition using genetically modified mice reveal the contribution of target genes to the olfactory processing. Here, we describe the olfactory avoidance test to investigate the odor detection ability in mice.
Keywords: OlfactionBackground
Olfactory system is a good model for studying the sensory processing in the brain. To characterize innate fear responses such as freezing and avoidance in genetically modified mice, the olfactory avoidance test was performed using a component of fox feces, TMT (2,5-dihydro-2,4,5-trimethylthiazoline; Kobayakawa et al., 2007). Furthermore, the olfactory avoidance using the different amounts of TMT was carried out to know the odor detection threshold in gene-knockout mice (Kaneko-Goto et al., 2013). Recently, we have reported that non-dihydrogenated TMT (nTMT: 2,4,5-trimethylthiazole) also induces similar freezing and avoidance responses (Takahashi et al., 2016). Here, we describe a method for the olfactory avoidance test with nTMT (commercially available) to explore the odor detection threshold in mice. This method has an advantage in the point using a simple device such as cage and filter paper, compare with that using an olfactometer.
Materials and Reagents
- Latex gloves (NIPPON Genetics, catalog number: SLPF-M )
- Filter paper (3 MM CHR) (GE Healthcare, catalog number: 3030-909 )
- Paper towels (KCWW, Kimberly-Clark, catalog number: 47000 )
- Laboratory-bred mice
Note: For the test, male mice should be used to avoid the effect of the estrous cycle. Mice are housed in groups 3-5 per cage, kept in a room with controlled temperature (~23 °C) and humidity under 12 h light/dark cycle (lights on at 8:30 AM) with ad libitum access to food and water. - 2,4,5-trimethylthiazole (nTMT) (7.9 M) (Tokyo Chemical Industry, catalog number: T1068 )
Note: A component of fox feces, TMT (2,5-dihydro-2,4,5-trimethylthiazoline), is known to evoke innate fear responses in rodents (Kobayakawa et al., 2007; Kaneko-Goto et al., 2013). Non-dihydrogenated TMT (nTMT) also induces similar freezing and avoidance responses (Takahashi et al., 2016). - 70% ethanol
Equipment
- Test cage (31 x 21 x 12.5 cm) (one cage per one mouse)
- Habituation cage with the same size as the test cage (four cages per one mouse)
- Clear acrylic board (which can cover the roof of the test cage)
- Video camera (Sony, catalog number: HDR-CX560V )
Note: The mouse behavior is recorded under the weak-light condition. This model (Sony Nightshot Camcorder) is equipped with infrared-mode. - Tripod for camera (SLIK, catalog number: F 740 )
- Red light
- Fume hood
Software
- Microsoft Excel (Microsoft)
Procedure
- Before the test day
- During five days before the test, mice are habituated to the experimental condition, under which an experimenter handles them with latex gloves on. The same experimenter gently handles each mouse for 10 min per day during the five consecutive days.
- On the test day (Figure 1)
- Tests are done during the dark phase of 12 h light/dark cycle in the test room, in which, the weak red light (< 5 lux) is used for indirect lighting. Individual mice are transferred from their home cage to each habituation cage in the habituation room. The mice are habituated to the experimental environment in the habituation cage for 30 min (Habituation phase). We recommend that the test is performed in the dedicated room, which is different from the habituation room, because it is important to prevent the mice from encountering nTMT before the test.
- Each mouse is transferred from an old to a new habituation cage. This habituation process is repeated four times for each animal (use four habituation cages per one mouse, totally for 2 h) (Habituation phase; Figure 1A, top).
- Set the test cage and a digital video camera in the test room. Put on a filter paper (2 x 2 cm) at the one side of cage. Cover the roof of the test cage with acrylic board to prevent the odor from diffusing and the mouse from escaping from the test cage (Figure 1B).
- Undiluted nTMT at the amount of 0, 0.4, 4, and 40 μl is dropped on the filter paper in the test cage.
- After the mouse is transferred to the test cage, record the behavior with a video camera during the 10-min test (Test phase; Figure 1A, bottom).
- The tested mouse is returned to their home cage. After the one test finishes, the used cage and filter paper scented with nTMT should be kept in the fume hood (see Note 4). Start the next test using new test cage and another mouse.
- The recorded videos are analyzed, as described below.
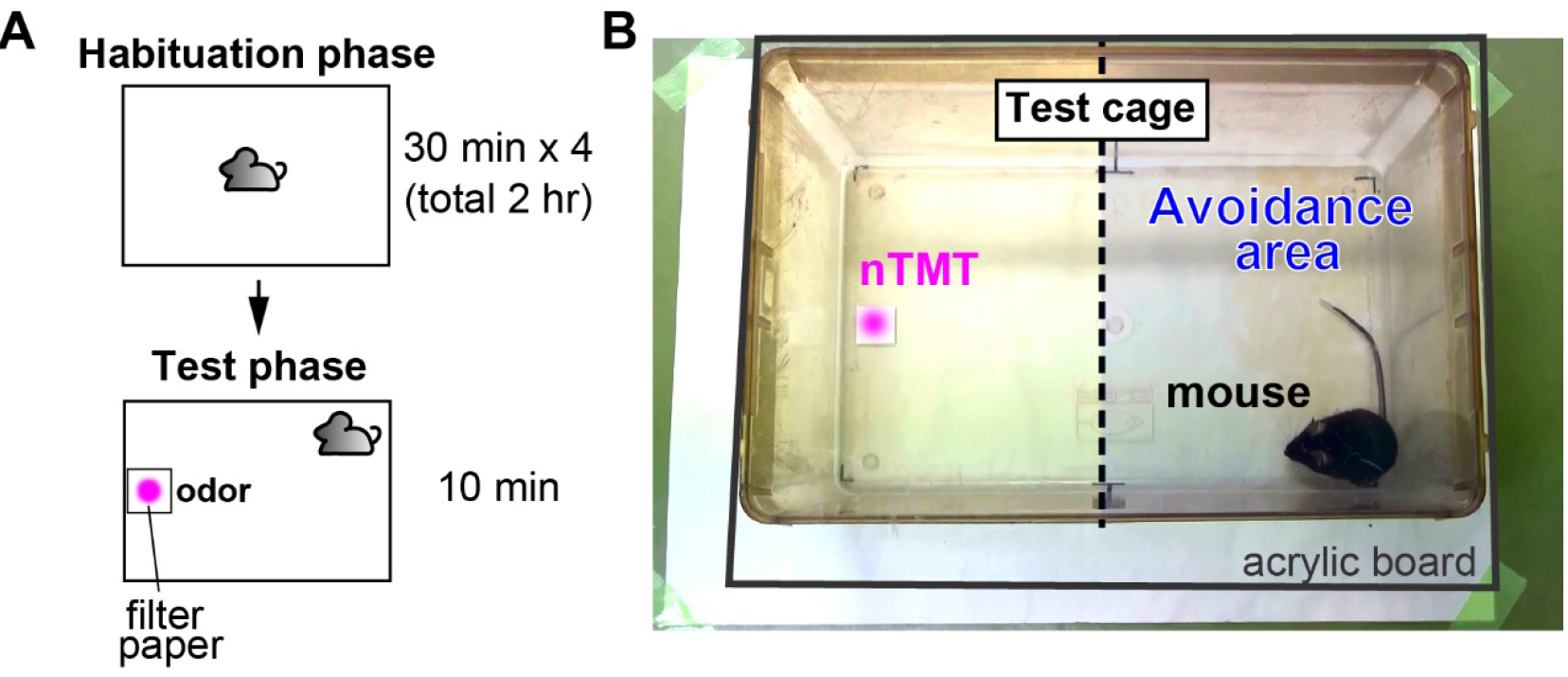
Figure 1. Olfactory avoidance test. A. Schema of the experimental procedure; B. Apparatus of the olfactory avoidance test.
- Tests are done during the dark phase of 12 h light/dark cycle in the test room, in which, the weak red light (< 5 lux) is used for indirect lighting. Individual mice are transferred from their home cage to each habituation cage in the habituation room. The mice are habituated to the experimental environment in the habituation cage for 30 min (Habituation phase). We recommend that the test is performed in the dedicated room, which is different from the habituation room, because it is important to prevent the mice from encountering nTMT before the test.
Data analysis
Both freezing and avoidance times are measured manually during the 10-min test using the recorded videos. ‘Freezing time’ is defined as the time kept still for more than 3 sec, except for breathing (Figure 2A and Video 1). ‘Avoidance time’ is defined as the time spent in an area without a filter paper scented with nTMT, when the test cage is divided into two equal areas. Avoidance behavior is represented by an avoidance index (avoidance index = [P - 50]/50, where P is the percentage of avoidance time during the 10-min test period; Figure 2B). We recommend that both freezing and avoidance times are measured by blinded analysis.
P-values are calculated by Welch t-test using Microsoft Excel, in which you click Data Analysis and perform t-test: Two-Sample Assuming Unequal Variances. For multiple pairwise comparisons, P-values are then sequentially evaluated according to the Holm-Bonferroni method (Holm, 1979) to keep an experiment-wise α ≤ 0.05, manually.
The formula to evaluate the Holm-Bonferroni method is as follows:
α/(n - k + 1)
Where,
n: number of tests,
k: rank number of pair.
Example for the Holm-Bonferroni correction:
Consider four null hypotheses (H1-4) with unadjusted P-values (p1-4), to be tested at significance level α = 0.05. H1: p1 = 0.01, H2: p2 = 0.003, H3: p3 = 0.03, H4: p4 = 0.04
- Order the P-values from smallest to largest.
p2 (= 0.003) < p1 (= 0.01) < p3 (= 0.03) < p4 (= 0.04) - To calculate the adjusted alpha level, work the Holm-Bonferroni formula for the first rank, and compare it with the first-ranked P-value (p2). If the P-value is smaller, reject the first-ranked null hypothesis (H2).
α/(n - k + 1) = 0.05/(4 - 1 + 1) = 0.0125
0.0125 > p2 (= 0.003). H2 is rejected. - Repeat the Holm-Bonferroni formula for the second rank, and compare it with the second-ranked P-value (p1).
α/(n - k + 1) = 0.05/(4 - 2 + 1) = 0.0167
0.0167 > p1 (= 0.01). H1 is rejected. - Repeat the Holm-Bonferroni formula for the third rank, and compare it with the third-ranked P-value (p3).
α/(n - k + 1) = 0.05/(4 - 3 + 1) = 0.0250
0.0250 < p3 (= 0.03). H3 is not rejected. - The testing stops when you reach the first non-rejected hypothesis. All subsequent hypotheses are non-significant. We conclude that H1 and H2 are rejected and H3 and H4 are not rejected.
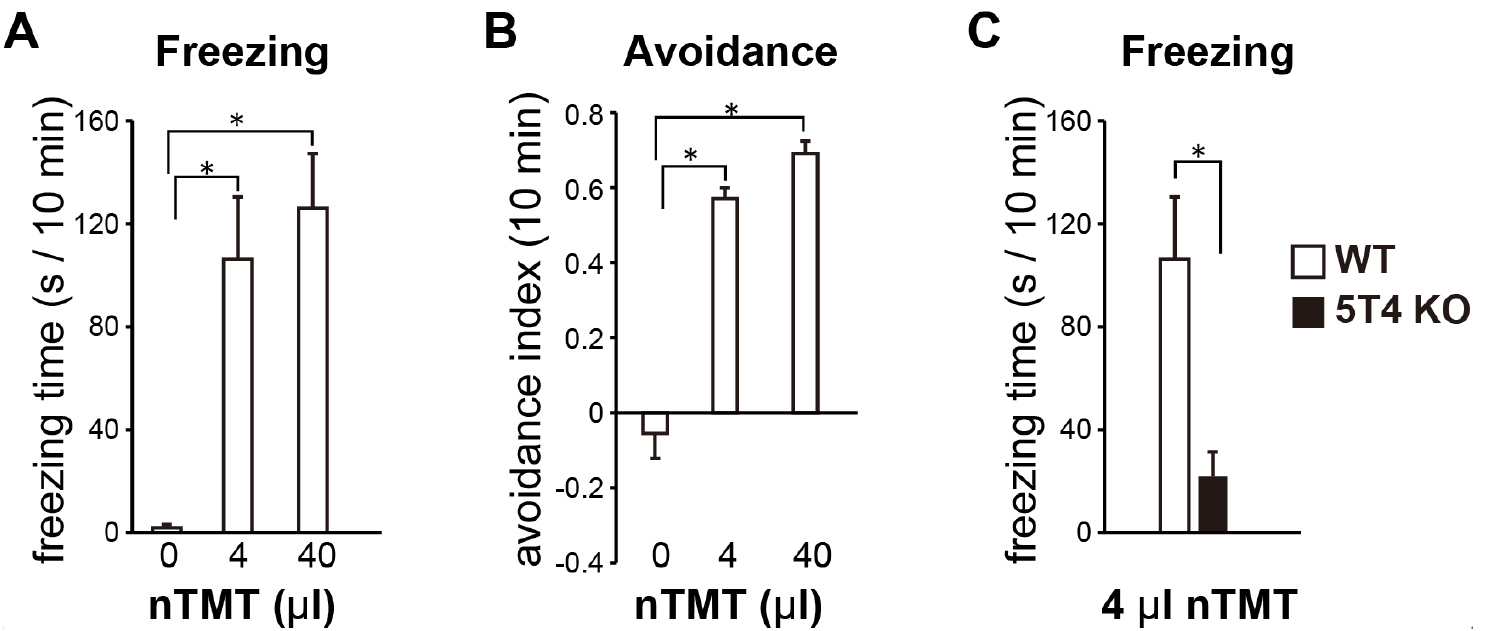
Figure 2. Sample data of the olfactory avoidance test. A and B. Freezing time (A) and avoidance index (B) in wild-type mice; C. When a lower amount (4 μl) of nTMT was employed, 5T4 knockout mice remarkably reduced the freezing time, compared with wild-type mice (see the detail in Takahashi et al., 2016).
Video 1. Freezing response in wild-type mice for 40 μl nTMT
Notes
- For this test, mice should be used only once to avoid confounding of data, based on the learning and memory.
- The experimenter must restrict from wearing odorant products with strong smell and from making any excessive noise during the test. Because it is difficult to completely remove the personal smell from the experimenter, we recommend that the same experimenter, who handles the mice for the habituation before the test, performs all the processes throughout the test.
- It is important to prevent the odor diffusion in the test room, because the undiluted odor is used in this test. The bottle of nTMT and their derivatives should be preserved in the fume hood. After the one test finishes, the used cage and filter paper scented with nTMT should be kept in the fume hood. In the test, a smaller amount of nTMT should be used first, compared with the larger amount. We recommend that the test is performed in the dedicated room, which is different from the habituation room, because it is important to prevent the mice from encountering nTMT before the test.
- The existence of experimenter affects the mouse behaviors in a significant way. The experimenter must depart enough from the test cage. We recommend that when the test phase starts in the dedicated test room, the experimenter gets out there.
Acknowledgments
This protocol was adopted from previous studies (Kobayakawa et al., 2007; Kaneko-Goto et al., 2013). This work was supported by Grants-in-Aid for Scientific Research on (B) (A.T.), (C) (H.T.), and Innovative Areas (Adaptive circuit shift) (A.T.), and for Challenging Exploratory Research (A.T.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scand J Statist 6(2): 65-70.
- Kaneko-Goto, T., Sato, Y., Katada, S., Kinameri, E., Yoshihara, S., Nishiyori, A., Kimura, M., Fujita, H., Touhara, K., Reed, R. R. and Yoshihara, Y. (2013). Goofy coordinates the acuity of olfactory signaling. J Neurosci 33(32): 12987-12996.
- Kobayakawa, K., Kobayakawa, R., Matsumoto, H., Oka, Y., Imai, T., Ikawa, M., Okabe, M., Ikeda, T., Itohara, S., Kikusui, T., Mori, K. and Sakano, H. (2007). Innate versus learned odour processing in the mouse olfactory bulb. Nature 450(7169): 503-508.
- Takahashi, H., Ogawa, Y., Yoshihara, S., Asahina, R., Kinoshita, M., Kitano, T., Kitsuki, M., Tatsumi, K., Okuda, M., Tatsumi, K., Wanaka, A., Hirai, H., Stern, P. L. and Tsuboi, A. (2016). A subtype of olfactory bulb interneurons is required for odor detection and discrimination behaviors. J Neurosci 36(31): 8210-8227.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Takahashi, H. and Tsuboi, A. (2017). Olfactory Avoidance Test (Mouse). Bio-protocol 7(5): e2153. DOI: 10.21769/BioProtoc.2153.
-
Takahashi, H., Ogawa, Y., Yoshihara, S., Asahina, R., Kinoshita, M., Kitano, T., Kitsuki, M., Tatsumi, K., Okuda, M., Tatsumi, K., Wanaka, A., Hirai, H., Stern, P. L. and Tsuboi, A. (2016). A subtype of olfactory bulb interneurons is required for odor detection and discrimination behaviors. J Neurosci 36(31): 8210-8227.
Category
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link