- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Streamlined Method for the Preparation of Growth Factor-enriched Thermosensitive Hydrogels from Soft Tissue
Published: Vol 7, Iss 3, Feb 5, 2017 DOI: 10.21769/BioProtoc.2128 Views: 8925
Reviewed by: Antoine de MorreeFederica PisanoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1251 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 211 Views

Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
Adriana Bajetto [...] Tullio Florio
Feb 5, 2026 92 Views
Abstract
Hydrogels are an ideal medium for the expansion of cells in three dimensions. The ability to induce cell expansion and differentiation in a controlled manner is a key goal in tissue engineering. Here we describe a detailed method for producing hydrogels from soft tissues with an emphasis on adipose tissue. In this method, soluble, extractable proteins are recovered from the tissue and stored while the remaining insoluble tissue is processed and solubilised. Once the tissue has been sufficiently solubilised, the extracted proteins are added. The resulting product is a thermosensitive hydrogel with proteins representative of the native tissue. This method addresses common issues encountered when working with some biomaterials, such as high lipid content, DNA contamination, and finding an appropriate sterilisation method. Although the focus of this article is on adipose tissue, using this method we have produced hydrogels from other soft tissues including muscle, liver, and cardiac tissue.
Keywords: HydrogelBackground
The main goal of tissue engineering is to generate new tissue by providing the body with a scaffold possessing similar properties to those of the target site. This allows for optimal remodeling and enables formation of de novo endogenous tissue. In the field of adipose tissue engineering, biomaterials derived from adipose tissue are of particular interest because adipose tissue is widely available and in theory provides the best possible environment for induction of adipogenesis (Flynn et al., 2007; Flynn, 2010; Uriel et al., 2008; Choi et al., 2009; Young et al., 2011). It has been established that adipocytes secrete adipogenic factors (Li et al., 1998; Shillabeer et al., 1989; Shillabeer et al., 1990) and that conditioned medium produced from either adipocytes or excised adipose tissue is able to induce adipogenesis in vitro (Sarkanen et al., 2012).
The development of an injectable hydrogel with properties closely matching those of healthy adipose tissue would potentially be of great use in regenerative medicine. The ideal hydrogel would be acellular, contain proteins that are representative of those found in natural adipose tissue, be structurally capable of maintaining a space after implantation, and be capable of inducing adipose tissue growth (Cheung et al., 2014; Drury and Mooney, 2003). We have previously reported on the production of a thermoresponsive hydrogel from excised adipose tissue which was shown to be adipogenic both in vitro and in vivo (Poon et al., 2013). The gel induced adipogenic differentiation of adipose-derived stem cells in vitro and was capable of producing adipose tissue from 8 weeks post-implantation in the subcutaneous layer of the rat back (Debels et al., 2015).
As originally reported, our adipose-derived hydrogel used dispase to decellularise the tissue prior to extraction. Although dispase is capable of efficiently decellularising tissue (Uriel et al., 2008; Prasertsung et al., 2008), we have since observed the degree of digestion varies greatly from batch to batch due to differences in tissue surface area. Additionally, the slight differences in decellularisation led to variations in lipid content between batches which altered protein extraction efficiency and clarity of the final product. The washing and delipidation steps were also of concern as they increased variation of the gel’s final physical properties. Since our original publication, we have developed a practical and efficient method to replace these early processes.
Here we provide a detailed protocol for producing a soft tissue-derived hydrogel which addresses many of these concerns and reduces batch-to-batch variability. In our new method, proteins are extracted from the tissue first in order to retain as much soluble protein as possible for subsequent re-addition. Decellularisation by dispase digestion has been replaced with cold homogenisation and nuclease treatment. Dispase is capable of efficiently decellularising tissue; it works by cleaving fibronectin and collagen IV, but there is a problem, these proteins may provide important functional groups which would otherwise be lost after dispase digestion (Gregoire et al., 1998; Khoshnoodi et al., 2008). Delipidation is no longer performed over the course of multiple salt washes and centrifugation steps, now it is done as part of the homogenisation and solubilisation steps. This method has been used to successfully produce thermoresponsive hydrogels from multiple tissues including skeletal muscle and organs such as the liver and heart. These hydrogels containing a collection of soluble proteins present in the native tissues may provide others in the field the basis for further developments in biomaterials research.
Materials and Reagents
- Whatman glass fibre filter paper (Sigma-Aldrich, catalog number: WHA1820021 )
- Spectrum Laboratories 12-14 kDa dialysis membrane (Pacific Laboratory Products, catalog number: 132706 )
- Freshly harvested subcutaneous porcine adipose tissue, liver, skin, cardiac tissue, and visceral fat (Donated by Diamond Valley Pork [Laverton North, VIC, Australia])
Note: All tissue not used immediately was stored at -20 °C. - Human tissues (Collected from fully consented patients with ethics approval from the St Vincent’s Hospital Melbourne Human Research Ethics Committee [Protocol 52/03])
Note: All tissue not used immediately was stored at -20 °C. - Protease inhibitors (Sigma-Aldrich)
- Ammonium sulphate (Sigma-Aldrich, catalog number: 31119 )
- 70% ethanol (Sigma-Aldrich)
- Spectrum Laboratories Spectra/Gel Absorbent (Pacific Laboratory Products, catalog number: 292600 )
- PBS (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
- DNeasy Blood & Tissue Kit (QIAGEN, catalog number: 69504 )
- PierceTM BCA Protein Assay Kit (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 23225 )
- Dulbecco’s modified Eagle medium (Sigma-Aldrich, catalog number: D5796 )
- Fetal calf serum (CSL, Australia)
- Antibiotics
- Oil Red O (Sigma-Aldrich, catalog number: O0625 )
- Haematoxylin
- Collagenase I
- 4% paraformaldehyde or 10% neutral-buffered formalin
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: 798681 )
- N-ethylmaleimide (NEM) (Sigma-Aldrich, catalog number: E3876 )
- Benzamidine hydrochloride hydrate (Sigma-Aldrich, catalog number: B6506 )
- Urea (Sigma-Aldrich, catalog number: U1250 )
- Guanidine hydrochloride (GuHCl) (Sigma-Aldrich, catalog number: 50950 )
- Glacial acetic acid (Sigma-Aldrich)
- Tris base (Sigma-Aldrich, catalog number: RDD008 )
- NaCl
- DNase I (Roche Diagnostics, catalog number: 11284932001 )
- RNase A (Roche Diagnostics, RNASEA-RO )
- MgCl2
- Zinc sulfate heptahydrate (ZnSO4·7H2O) (Sigma-Aldrich, catalog number: Z0251 )
- Chloroform (Sigma-Aldrich)
- Methanol (Sigma-Aldrich)
- Pepsin (Worthington Biochemical, catalog number: LS003317 )
- Solutions (Prepared according to the directions outlined in Recipes)
- 0.5 M ethylenediaminetetraacetic acid (EDTA)
- 50 mg/ml N-ethylmaleimide (NEM)
- 50 mg/ml benzamidine hydrochloride hydrate
- 8 M urea
- 8 M guanidine hydrochloride (GuHCl)
- 0.5 N acetic acid
- 4 M GuHCl extraction buffer
- 10x Tris-buffered saline (TBS)
- Nuclease solution
- 1,000x haemoglobin precipitation solution
- Chloroform-methanol lipid extraction solution
- 10x protease inhibitors
- 0.75% pepsin
Equipment
- Knife or scalpel
- Balance
- 4 L beaker
- Rotary mixer
- Shaking incubator
- Centrifuge (capable of speeds of 15,000 x g)
- Food processor, immersion blender, or high capacity tissue homogeniser
- Cheesecloth or fine mesh gauze
- Humidified 37 °C incubator with 5% CO2 (for in vitro testing)
- Fume hood
Software
- ImageJ (https://imagej.nih.gov/ij/)
- GraphPad Prism 6 (GraphPad Software, Inc.)
Procedure
- Dissection
Using a sharp knife or scalpel, shave frozen tissue into 2-3 mm thick pieces and weigh on a balance. Freezing the tissue serves two purposes - to lyse the cells and to make slicing easier. Freshly harvested tissue may also be used but care must be taken to keep the tissue cold. The final yield of hydrogel will be 1-3x the volume of the tissue you are processing.
Notes:- Freshly collected tissue, whether animal or human, may be processed immediately or stored at -20 °C to -80 °C for longer term storage prior to processing. Please note that tissue stored for extended periods (> 6 months) will result in hydrogels with reduced efficacy.
- For convenience, the weight of the tissue in the following steps is referred to as one (1x) volume.
- Freshly collected tissue, whether animal or human, may be processed immediately or stored at -20 °C to -80 °C for longer term storage prior to processing. Please note that tissue stored for extended periods (> 6 months) will result in hydrogels with reduced efficacy.
- Extraction
- Transfer the sliced tissue to an airtight container and add a minimum of 2x volumes (based on weight of tissue) of 4 M guanidine hydrochloride (GuHCl) extraction buffer. Gently mix at 4 °C for 16-48 h to allow for complete extraction of the tissue.
Note: The volume of GuHCl extraction buffer used depends on the tissue being processed; tissue with a high level of extractable protein will require larger volumes. Adjust the volume of GuHCl as required. In general, if the resulting protein extract is highly viscous, add an additional volume of extraction buffer and incubate for another 16 h for further extraction – tissue very high in collagen such as skin and tendon will have lower amounts of total extractable protein than other tissue such as muscle and soft organs like liver. Ideally, extraction steps should be done in an end-over-end manner with a rotary mixer to ensure the tissue is properly mixed during incubation. Alternative mixers may also be used provided they result in adequate movement of the tissue during extraction. The use of magnetic stirrers is not recommended. - After at least 16 h, collect the 4 M GuHCl containing the soluble protein extracted from the tissue and filter through glass fibre filter paper to remove particulate material.
Note: If the 4 M GuHCl protein extract does not easily pass through glass fibre filter paper, dilute it with 4 M GuHCl and try again. - Dialyse the protein extract against a total of 100x volumes of water at 4 °C.
a. Pour the 4 M GuHCl protein extract to a suitable length of dialysis tubing, being sure not to fill the tubing more than ~70% to allow for expansion during dialysis.
b. Transfer the dialysis tubing containing the protein extract to a pre-chilled 4 L beaker of water and mix at 4 °C.
c. Replace the water every 1-2 h until a total of ~100x volumes of water has been used for dialysis (the dialysis may be left overnight). - Take the leftover extracted tissue and wash in water for 1 h by gently mixing at 4 °C (change the water every 10 min). Once washed, begin processing this tissue as directed in the next section - Tissue processing.
Note: After completing steps B2-B4, you will have two samples - a liquid GuHCl fraction containing extracted proteins, and leftover pieces of extracted tissue. The liquid fraction is processed according to instructions outlined in steps B5-B8; the solid tissue is processed according to step C - Tissue Processing. - After dialysis in water, measure the volume of the GuHCl extract. Add 1 µl of 0.5 M zinc sulphate (ZnSO4) (1,000x stock) per ml of GuHCl extract, to begin precipitating haemoglobin (Hover and Kulkarni, 2000). Briefly mix, then incubate the protein extract at 4 °C for 30 min before centrifuging at 15,000 x g for 15 min at 4 °C.
- Transfer the supernatant to a clean container and add protease inhibitors (1 mM each of N-ethylmaleimide, benzamidine, and EDTA). Transfer the extract to dialysis membrane and dialyse overnight in water at 4 °C to remove excess ZnSO4.
- Pour the extract to a suitable length of dialysis tubing, being sure not to fill the tubing more than ~70% to allow for expansion during dialysis.
- Transfer the dialysis tubing containing the protein extract to a pre-chilled 4 L beaker of water and mix at 4 °C.
- Replace the water every 1-2 h until a total of ~100x volumes of water has been used for dialysis (the dialysis may be left overnight).
- Pour the extract to a suitable length of dialysis tubing, being sure not to fill the tubing more than ~70% to allow for expansion during dialysis.
- After dialysis, measure the volume of the protein extract and transfer to a beaker with a capacity at least double that of the extract volume. While stirring the extract on ice, slowly dissolve 0.5 g of ammonium sulphate per ml of tissue extract (Doonan, 1996). Once the ammonium sulphate has completely dissolved, allow the protein to precipitate at 4 °C for at least 1 h or preferably overnight.
- Once precipitated, collect the protein by centrifugation at 15,000 x g for 20 min at 4 °C. Discard the supernatant and dissolve the protein pellet in water. Use as little water as possible to completely dissolve the protein. Store at -20 °C until step D (final processing).
Note: The residual ammonium sulphate present in the pellet will help solubilise the protein. Although extracts stored for up to 12 months have been shown to retain much of their efficacy, it is preferable to limit the storage time prior to use.
- Transfer the sliced tissue to an airtight container and add a minimum of 2x volumes (based on weight of tissue) of 4 M guanidine hydrochloride (GuHCl) extraction buffer. Gently mix at 4 °C for 16-48 h to allow for complete extraction of the tissue.
- Tissue processing
- After washing the pieces of GuHCl-extracted tissue in water, re-weigh the tissue, then transfer to a large container and homogenise in an excess volume of chilled water (> 20x volumes).
Note: High capacity tissue homogenisers are difficult to find; commercially available food processors or immersion blenders may be used instead. If adipose tissue is being processed, it is important to thoroughly homogenise the tissue to release the lipid from the tissue. It is important that the water used in this step is chilled so that any lipid present in the tissue becomes solid. - Using forceps, remove a small amount of tissue, rinse thoroughly in a separate beaker of water and transfer to a clean container on ice (Figure 1). Repeat this process until all of the tissue has been collected. At this stage, it may be necessary to repeat this procedure if you are working with adipose tissue - homogenise in chilled water and repeat as necessary until all visible lipid is removed.
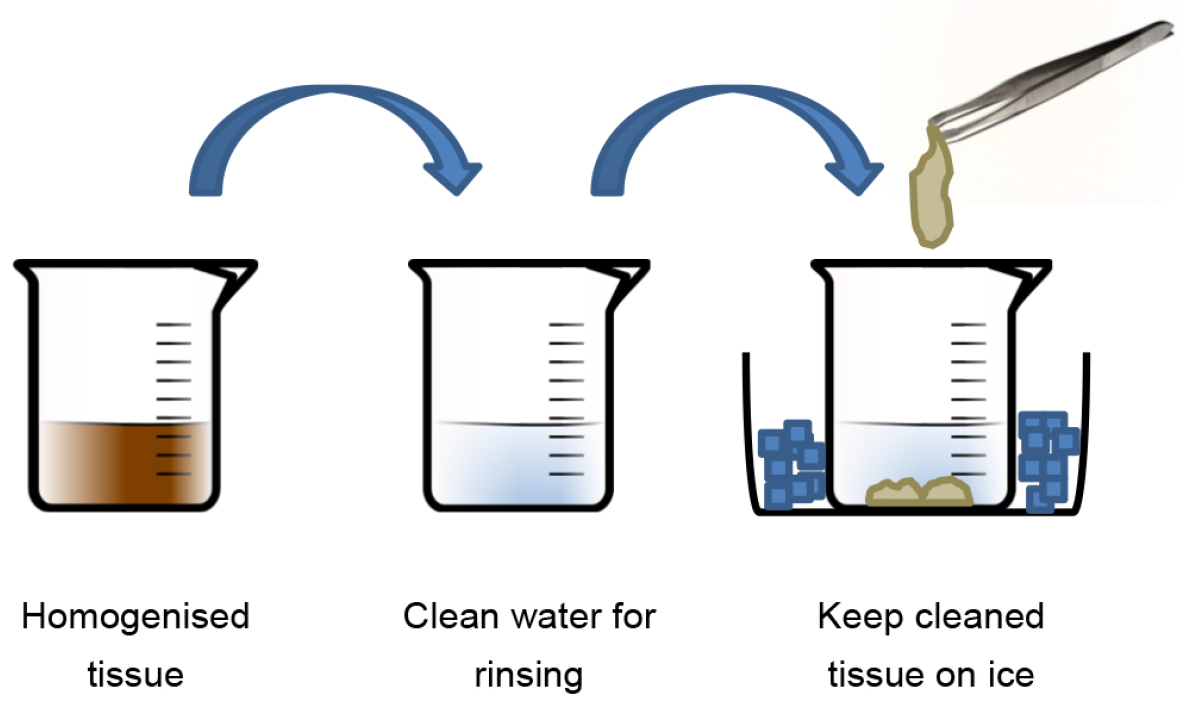
Figure 1. Procedure for cleaning homogenised tissue - Once washed, blot the tissue dry against absorbent paper and measure the weight - this is 1x volume.
- Prepare 1.5x volumes of nuclease buffer. Transfer the tissue and nuclease buffer to an airtight container and gently mix at 37 °C for at least 1 h.
- After digestion, discard the nuclease buffer and wash the tissue three times in water at room temperature.
- Discard the water and add at least 10x volumes of 70% ethanol. Mix at 37 °C for at least 15 min. Discard the ethanol, then wash the tissue three times with water at room temperature.
- For adipose or similarly fatty tissues, the Folch method of lipid extraction may be used at this stage to further remove any remaining lipid (Folch et al., 1957). Prepare 10-20x volumes of 2:1 (chloroform:methanol) and mix with the tissue for 20 min in a fume hood at room temperature. Discard the liquid waste appropriately, then wash the tissue thoroughly three or more times with an excess volume of water. Blot the tissue dry against absorbent paper and measure the weight - this is 1x volume.
- Prepare 2x volumes of 0.75% pepsin in 0.5 M acetic acid. Mix at room temperature until completely dissolved. Rinse the tissue briefly (30 sec) with an excess volume of 0.5 M acetic acid to pre-swell the tissue. Discard the acetic acid, transfer the tissue to an air-tight container, and add the pepsin. Mix vigorously at room temperature until the tissue has been digested (≥ 2 days - check the digest regularly). Be sure to incubate the tissue at room temperature; not 37 °C, and do not digest the tissue longer than is necessary. The digested tissue should be relatively clear and quite viscous.
Note: Alternatively, the tissue may be homogenised with an equal volume of 0.5 M acetic acid prior to adding the solubilised pepsin. This will greatly shorten the required digestion time, however the end product may require concentration before use. Depending on the tissue being digested, it may be necessary to add additional pepsin. Tissue very high in collagen such as skin and tendon may require the addition of more pepsin for complete digestion (Neuman et al., 1950). After 24 h, if the digest is especially viscous, additional 0.75% pepsin may be added until the tissue is able to be easily mixed. Care must be taken to only add the minimum amount of pepsin as necessary in order to reduce the potential for immunogenic reactions (Northrop, 1930; Samuels et al., 2009; Seastone et al., 1937). Mix until digested. - Once digested, remove any particulate matter by squeezing through cheesecloth, fine mesh gauze or the equivalent. Follow this with centrifugation at 15,000 x g, 4 °C, 20 min. Carefully remove any visible lipid at the surface and transfer the digested protein to dialysis membrane. Dialyse against 100x volumes of chilled TBS at 4 °C for 16-48 h to neutralise the pepsin.
Note: Use a spatula to collect all of the digested protein from the cheesecloth. A lot of protein can be lost at this stage if you are not careful.
- After washing the pieces of GuHCl-extracted tissue in water, re-weigh the tissue, then transfer to a large container and homogenise in an excess volume of chilled water (> 20x volumes).
- Final processing
- Remove the liquid protein extract from -20 °C storage and thaw. Add all of the liquid extract to the neutralised pepsin digest and mix well. Transfer the mixture to dialysis membrane and begin sterilisation. At this stage, Spectra/Gel Absorbent may be used at 4 °C to increase the concentration of the gel prior to sterilisation (following the manufacturer’s instructions).
- Sterilise the gel by dialysis in chilled 8 M urea (Roberts and Lloyd, 2007) overnight at 4 °C, followed by dialysis overnight in chilled 0.5% chloroform/TBS at 4 °C.
Note: After dialysis in 8 M urea, all of the protein should be thoroughly solubilised and more concentrated. - Finally, dialyse against 100x volumes of chilled TBS for 16 h and finish with dialysis against 100x volumes of chilled PBS for 16 h. Store aliquots of gel at -20 °C to -80 °C. Working aliquots of hydrogel should be thawed slowly at 4 °C. Thawed hydrogels may be stored at 4 °C for one month without any loss of efficacy. Depending on the desired concentration, the final yield should be between 1-3x the starting volume of tissue. A sample of the final product should be assayed for DNA (QIAGEN DNeasy Blood & Tissue Kit) and protein concentration (PierceTM BCA Protein Assay Kit) before undergoing in vitro testing. Gelation should occur within 10 min at 37 °C. Sterile PBS may be used to adjust the final protein concentration if desired.
Note: Protein concentrations > 3 mg/ml are recommended to maintain integrity of the hydrogel after gelation, however gelation will occur at concentrations ~1 mg/ml.
- Remove the liquid protein extract from -20 °C storage and thaw. Add all of the liquid extract to the neutralised pepsin digest and mix well. Transfer the mixture to dialysis membrane and begin sterilisation. At this stage, Spectra/Gel Absorbent may be used at 4 °C to increase the concentration of the gel prior to sterilisation (following the manufacturer’s instructions).
Data analysis
- The manner in which the hydrogels are tested and analysed is entirely up to the discretion of the researcher, however the type of cells used for testing, the number of replicates, and the total number of cells used should be sufficient to give meaningful, consistent results. The general procedure described below for adipose-derived hydrogel testing may be adapted for the testing of other types of tissue-derived hydrogels.
- The following section expands on, and supplements, the analysis originally described in our previous article for the analysis of the adipogenic activity of adipose-derived hydrogels (Poon et al., 2013); the reader is advised to refer to that publication for experimental methodology and further details.
- In vitro-testing of adipogenic hydrogel is typically carried out using adipose-derived stem cells (ADSC) cultured in Dulbecco’s modified Eagle medium supplemented with 10% foetal calf serum and antibiotics. Cells are then either supplemented with a cocktail of adipogenic components (Zuk et al., 2001) or with the hydrogel test material. After a period of approximately two weeks, the hydrogel is removed from the culture dishes prior to fixation and staining with Oil Red O (90 min) and haematoxylin (3 min). In the cases where hydrogels are difficult to remove, a short digestion with collagenase I (2 mg/ml) will help to detach the gels. After thoroughly removing all traces of hydrogel from the culture dishes, the cells are fixed in formaldehyde (4% paraformaldehyde or 10% neutral-buffered formalin), stained with Oil Red O/haematoxylin, and then quantitated.
Note: Hydrogels can be pre-set on one edge of the tissue culture dish prior to cell seeding – pipette a volume of hydrogel in the dish and incubate at 37 °C at a 45° angle until the gel has fully set (~10 min). - Quantitation of the differentiated cells in our original publication was performed using the freely available software, ImageJ (https://imagej.nih.gov/ij/) (Rasband, 1997-2016; Abramoff et al., 2004).
- If the tissue culture wells are sufficiently small, or your photographic equipment is capable, it is advised to take a single, high resolution image of the entire culture surface; otherwise multiple randomly-selected fields should be photographed for each tissue culture well.
- Each of the images is opened in ImageJ and analysed as follows:
- Open image in ImageJ.
- Select Plugins→Analyze→Grid. Choose an appropriate colour for the grid lines and select OK (Figure 2).
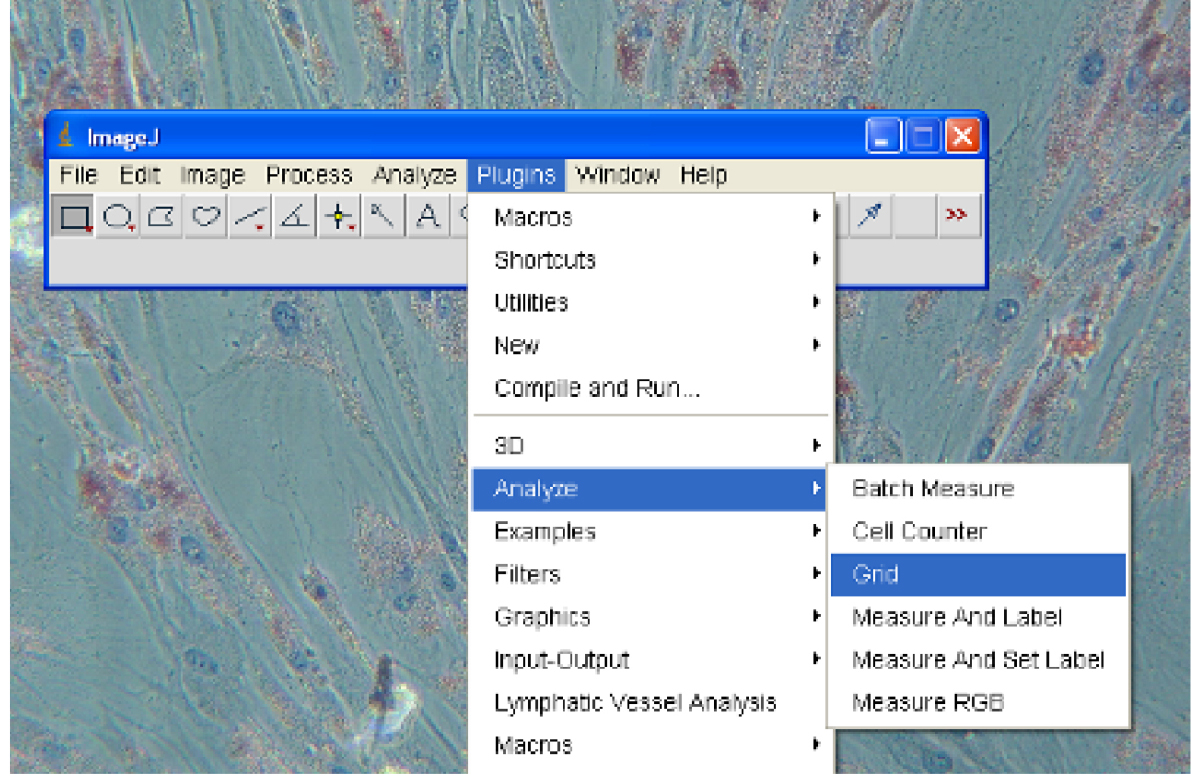
Figure 2. Turning on Grid lines - Select Plugins→Analyze→Cell Counter (Figure 3).
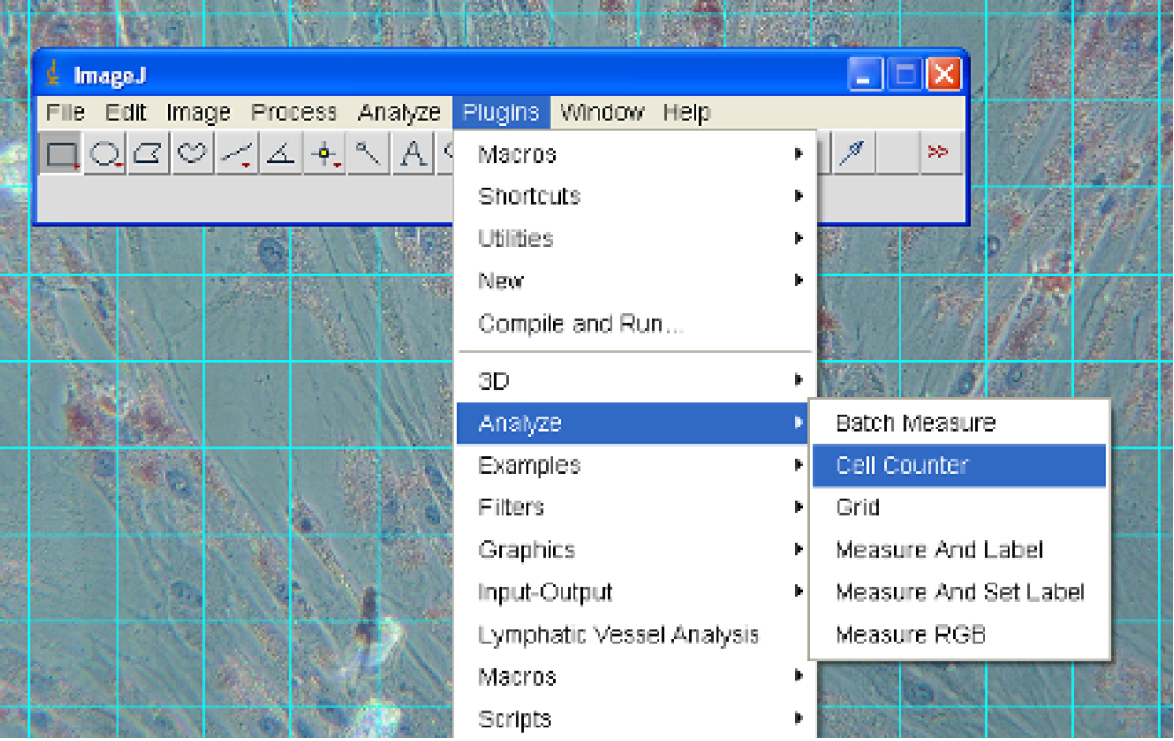
Figure 3. Selecting the Cell Counter
- In the Cell Counter window that appears, select ‘Initialize’, then select one of the 8 counters. Each counter has a different colour (Figure 4).
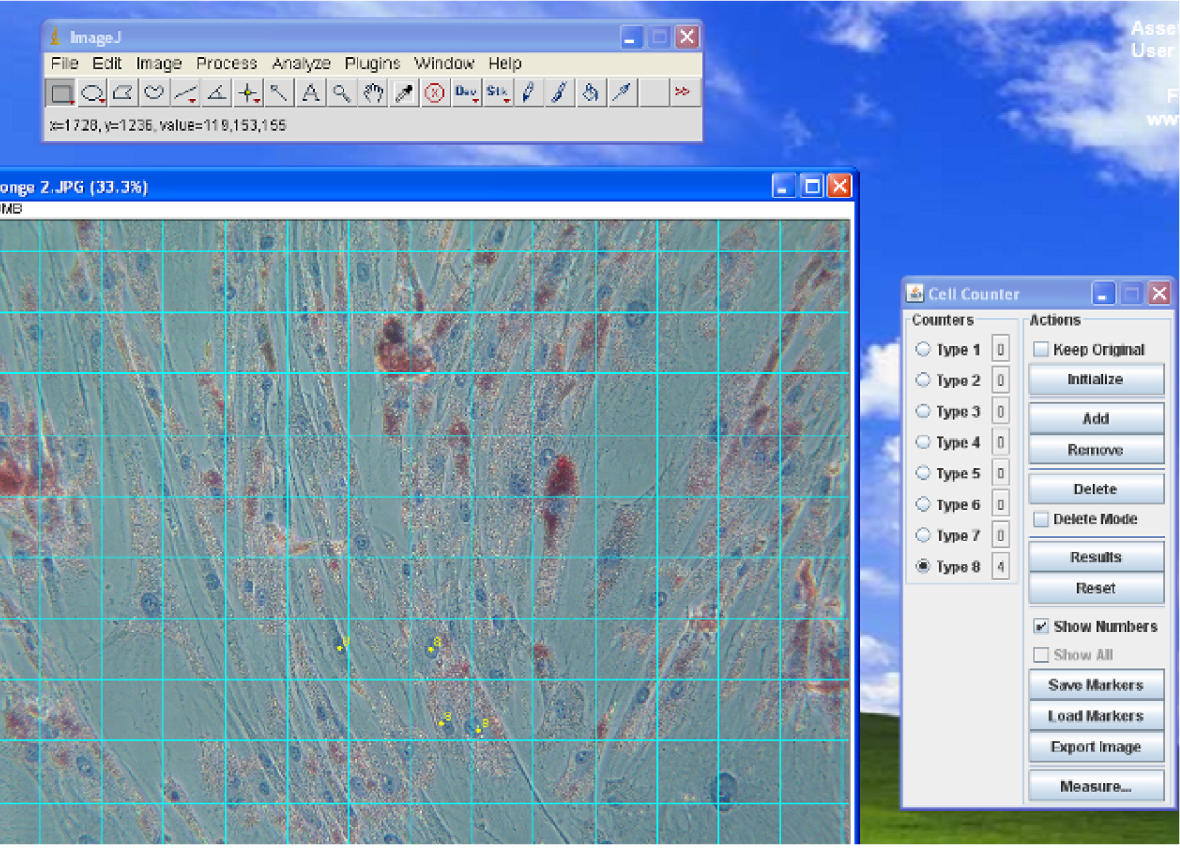
Figure 4. The Cell Counter window - Start by clicking on all of the haematoxylin-stained cells to get a total cell count, a running tally will appear in the window next to the counter number. Now choose another counter and click on all of the Oil Red O-stained cells.
- Once you have finished your counting, select Save Markers from the window to save your results.
- The proportion of Oil Red O-stained cells can be determined from these two numbers.
- After quantitating all of the images taken from the in vitro analysis, a table of figures can be produced for analysis. The numbers shown in Table 1 were the result of quantitation done for our previous publication (Poon et al., 2013). Statistical analysis by one way analysis of variance with post-hoc Tukey’s multiple comparisons test was performed using GraphPad Prism 6 (GraphPad Software, Inc.) to compare the adipogenic activity of each test condition.Table 1. Quantitation of adipocytes. Human ADSCs were supplemented with adipogenic medium (Zuk), adipose-derived hydrogel (Gel), or adipogenic medium and adipose-derived hydrogel (Gel + Zuk), then stained with Oil Red O and haematoxylin, and quantitated.
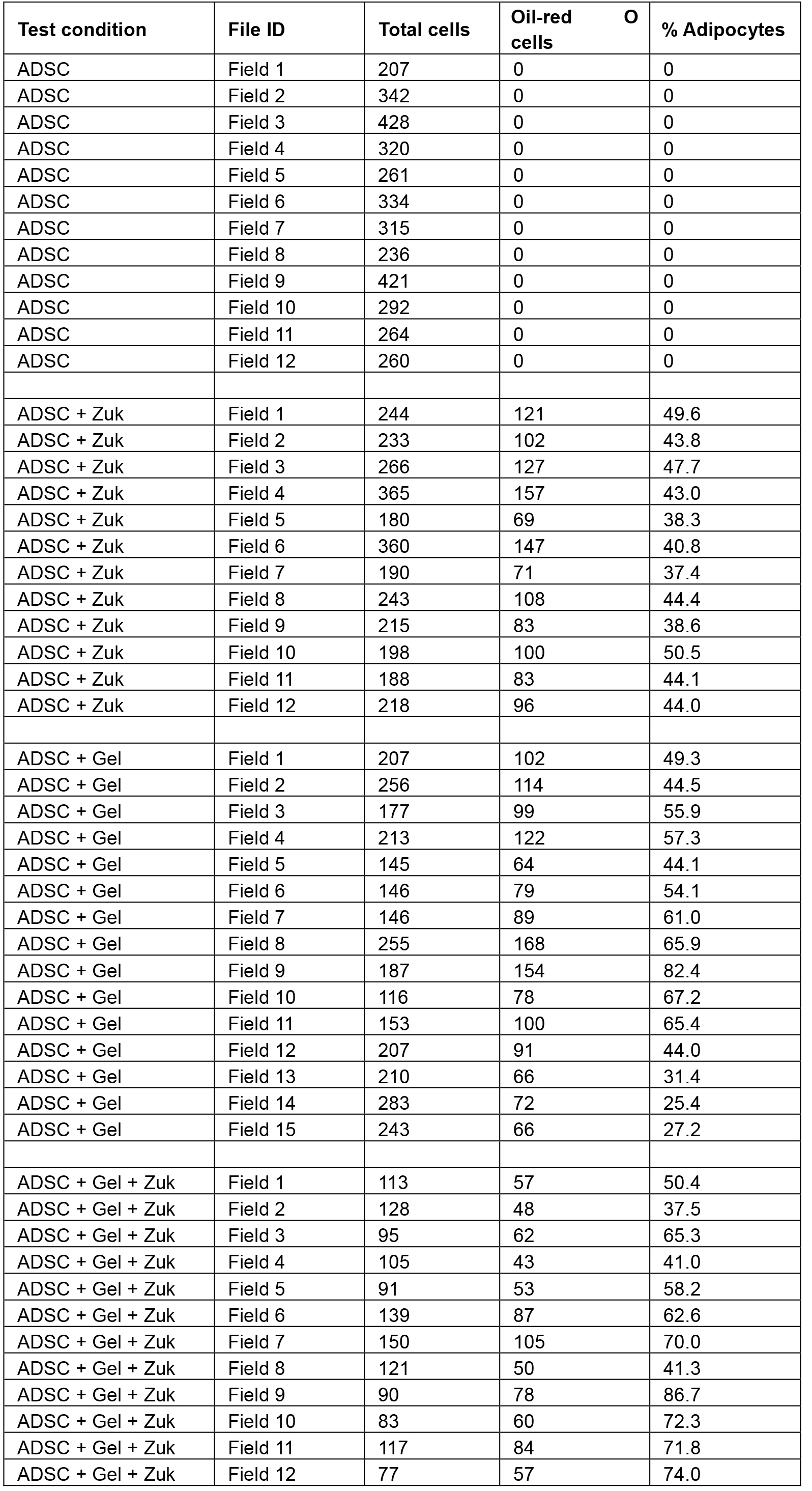
The final result of the in vitro analysis is shown in Table 2 and Figure 5 below; a version of this figure was presented previously (Poon et al., 2013).
Table 2. Summary of statistical analysis by one way ANOVA with post-hoc Tukey’s multiple comparisons test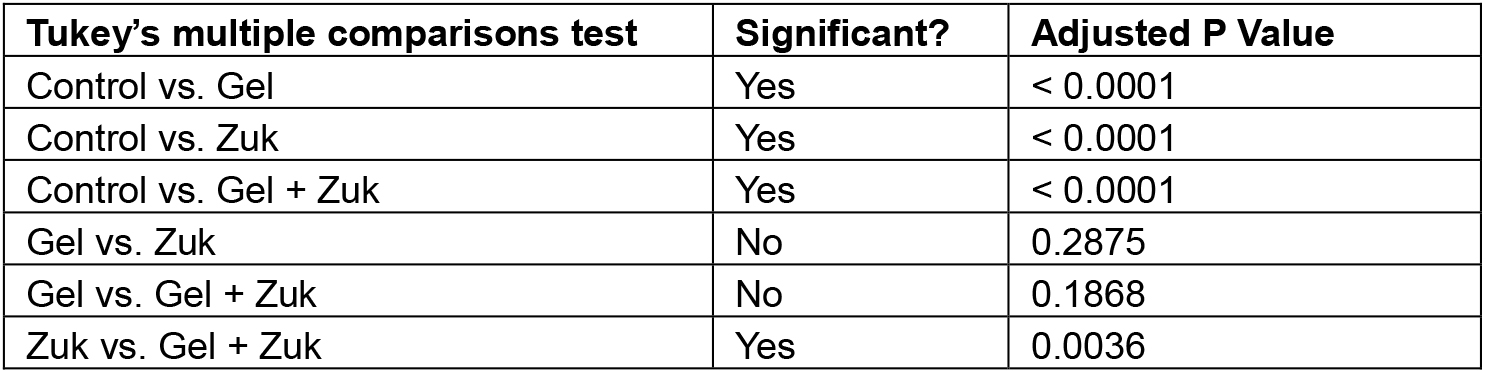
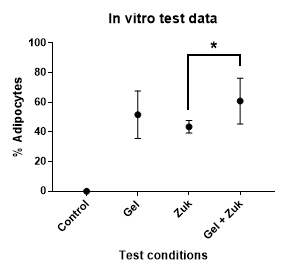
Figure 5. The adipogenic activity of adipose-derived hydrogel versus Zuk’s adipogenic medium on human adipose-derived stem cells. The data shows the mean with SD. Statistical analysis by one way ANOVA shows a statistically significant increase in adipogenic activity when Zuk’s adipogenic medium is supplemented with adipose-derived hydrogel (*). This analysis was described previously (Poon et al., 2013).With adipose-derived hydrogels, we have observed some batch-to-batch variation in adipogenic activity. The variables responsible for this are unknown, but age appears to be an important factor. If possible, it is advisable to screen fat samples from multiple tissue sources for adipogenic activity prior to large scale production. Conditioned medium can be produced from tissue samples by incubating the fat in ten volumes of complete medium under standard tissue culture conditions for 24 h. Once filter-sterilised, the conditioned medium can be tested for adipogenic activity on ADSCs. The most adipogenic tissue as determined by this screening process can be used for hydrogel production.
Recipes
- 0.5 M ethylenediaminetetraacetic acid (EDTA) (250 ml)
36.53 g of EDTA
Adjust pH to 8
Add double distilled water to 250 ml - 50 mg/ml N-ethylmaleimide (NEM) (2.5 M; 10 ml)
0.5 g of NEM
Ethanol to 10 ml - 50 mg/ml benzamidine hydrochloride hydrate (3.13 M; 1 ml)
50 mg of benzamidine HCl
Add double distilled water to 1 ml - 8 M urea (1 L)
480.48 g of urea
Add double distilled water to 1 L
Filter through glass fibre filter paper - 8 M guanidine hydrochloride (GuHCl) (1 L)
764.32 g of GuHCl
Add double distilled water to 1 L
Filter through glass fibre filter paper - 0.5 N acetic acid (1 L)
971.2 ml of double distilled water
28.8 ml of glacial acetic acid - 4 M GuHCl extraction buffer (50 mM Tris-HCl pH 7.4; 4 M GuHCl; 100 ml)
5 ml of 1 M Tris-HCl pH 7.4
50 ml of 8 M GuHCl
Add double distilled water to 100 ml - 10x Tris-buffered saline (TBS) (0.5 M Tris-HCl; 1.5 M NaCl; 1 L)
60.57 g of Tris
87.66 g of NaCl
Adjust pH to 7.4
Add double distilled water to 1 L
Filter through glass fibre filter paper - Nuclease solution (50 µg/ml DNase I; 10 µg/ml RNase A; 10 mM MgCl2; 10 ml)
50 µl of 10 mg/ml DNase I
10 µl of 10 mg/ml RNase A
100 µl of 1 M MgCl2
PBS to 10 ml - 1,000x haemoglobin precipitation solution (0.5 M ZnSO4; 50 ml)
7.2 g of ZnSO4·7H2O
Add double distilled water to 50 ml - Chloroform-methanol lipid extraction solution ([2:1] chloroform:methanol; 150 ml)
100 ml of chloroform
50 ml of methanol - 10x protease inhibitors (10 mM EDTA pH 8; 10 mM N-ethylmaleimide; 10 mM benzamidine; 100 ml)
2 ml of 0.5 M EDTA pH 8
400 µl of 50 mg/ml NEM
319 µl of 50 mg/ml benzamidine HCl
Add double distilled water to 100 ml - 0.75% pepsin (7.5 mg/ml pepsin; 50 ml)
375 mg of pepsin
0.5 M acetic acid to 50 ml
Acknowledgments
We thank Dr. Kiryu K. Yap (St Vincent’s Institute and University of Melbourne, Department of Surgery) for editorial assistance with the manuscript. This work was supported by the Victorian State Government OIS Program and National Health and Medical Research Council Project Grant 1064786.
References
- Abramoff, M. D., Magelhaes, P. J. and Ram, S. J. (2004). Image processing with ImageJ. Biophotonics International 11(7): 36-42.
- Choi, J. S., Yang, H. J., Kim, B. S., Kim, J. D., Kim, J. Y., Yoo, B., Park, K., Lee, H. Y. and Cho, Y. W. (2009). Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J Control Release 139(1): 2-7.
- Cheung, H. K., Han, T. T., Marecak, D. M., Watkins, J. F., Amsden, B. G. and Flynn, L. E. (2014). Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 35(6): 1914-1923.
- Debels, H., Gerrand, Y. W., Poon, C. J., Abberton, K. M., Morrison, W. A. and Mitchell, G. M. (2015). An adipogenic gel for surgical reconstruction of the subcutaneous fat layer in a rat model. J Tissue Eng Regen Med 5(6).
- Doonan, S. (1996). Concentration of extracts. Methods Mol Biol 59: 95-102.
- Drury, J. L. and Mooney, D. J. (2003). Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24(24): 4337-4351.
- Flynn, L. E. (2010). The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 31(17): 4715-4724.
- Flynn, L., Prestwich, G. D., Semple, J. L. and Woodhouse, K. A. (2007). Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials 28(26): 3834-3842.
- Folch, J., Lees, M. and Sloane Stanley, G. H. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1): 497-509.
- Gregoire, F. M., Smas, C. M. and Sul, H. S. (1998). Understanding adipocyte differentiation. Physiol Rev 78(3): 783-809.
- Hover, C. G. and Kulkarni, A. P. (2000). A simple and efficient method for hemoglobin removal from mammalian tissue cytosol by zinc sulfate and its application to the study of lipoxygenase. Prostaglandins Leukot Essent Fatty Acids 62(2): 97-105.
- Khoshnoodi, J., Pedchenko, V. and Hudson, B. G. (2008). Mammalian collagen IV. Microsc Res Tech 71(5): 357-370.
- Li, Z. H., Carraro, R., Gregerman, R. I. and Lau, D. C. (1998). Adipocyte differentiation factor (ADF): a protein secreted by mature fat cells that induces preadipocyte differentiation in culture. Cell Biol Int 22(4): 253-270.
- Neuman, R. E. and Logan, M. A. (1950). The determination of collagen and elastin in tissues. J Biol Chem 186(2): 549-556.
- Northrop, J. H. (1930). Crystalline pepsin: I. Isolation and tests of purity. J Gen Physiol 13(6): 739-766.
- Poon, C. J., Pereira, E. C. M. V., Sinha, S., Palmer, J. A., Woods, A. A., Morrison, W. A. and Abberton, K. M. (2013). Preparation of an adipogenic hydrogel from subcutaneous adipose tissue. Acta Biomater 9(3): 5609-5620.
- Prasertsung, I., Kanokpanont, S., Bunaprasert, T., Thanakit, V. and Damrongsakkul, S. (2008). Development of acellular dermis from porcine skin using periodic pressurized technique. J Biomed Mater Res B Appl Biomater 85(1): 210-219.
- Rasband, W. S. (1997-2016). ImageJ. National Institutes of Health, Bethesda, Maryland, USA.
- Roberts, P. L. and Lloyd, D. (2007). Virus inactivation by protein denaturants used in affinity chromatography. Biologicals 35(4): 343-347.
- Samuels, T. L. and Johnston, N. (2009). Pepsin as a causal agent of inflammation during nonacidic reflux. Otolaryngology Head Neck Surg 141(5): 559-563.
- Sarkanen, J. R., Kaila, V., Mannerstrom, B., Raty, S., Kuokkanen, H., Miettinen, S. and Ylikomi, T. (2012). Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng Part A 18(1-2): 17-25.
- Seastone, C. V. and Herriott, R. M. (1937). Immunological studies on pepsin and pepsinogen. J Gen Physiol 20(6): 797-806.
- Shillabeer, G., Forden, J. M. and Lau, D. C. (1989). Induction of preadipocyte differentiation by mature fat cells in the rat. J Clin Invest 84(2): 381-387.
- Uriel, S., Huang, J. J., Moya, M. L., Francis, M. E., Wang, R., Chang, S. Y., Cheng, M. H. and Brey, E. M. (2008). The role of adipose protein derived hydrogels in adipogenesis. Biomaterials 29(27): 3712-3719.
- Young, D. A., Ibrahim, D. O., Hu, D. and Christman, K. L. (2011). Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater 7(3): 1040-1049.
- Zuk, P. A., Zhu, M., Mizuno, H. Huang, J., Futrell, J. W., Katz, A. J., Benhaim, P., Lorenz, H. P., Hedrick, M. H. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2): 211-228.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Poon, C. J., Tan, S. S., Boodhun, S. W., Abberton, K. M. and Morrison, W. A. (2017). A Streamlined Method for the Preparation of Growth Factor-enriched Thermosensitive Hydrogels from Soft Tissue. Bio-protocol 7(3): e2128. DOI: 10.21769/BioProtoc.2128.
Category
Stem Cell > Adult stem cell > Maintenance and differentiation
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









