- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Electro-fusion of Gametes and Subsequent Culture of Zygotes in Rice
Published: Vol 6, Iss 24, Dec 20, 2016 DOI: 10.21769/BioProtoc.2074 Views: 12545
Reviewed by: Renate WeizbauerYvon JaillaisAlizée Malnoe

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
Jason T. Roberts [...] David M. Braun
Oct 20, 2023 2240 Views

Live Imaging of the Shoot Apical Meristem of Intact, Soil-Grown, Flowering Arabidopsis Plants
Gabriele Bradamante
Jun 20, 2024 2272 Views

Micrografting Technique of Hevea brasiliensis In Vitro Plantlets
Florence Dessailly [...] Julie Leclercq
Feb 20, 2025 1479 Views
Abstract
Electro-fusion system with isolated gametes has been utilized to dissect fertilization-induced events in angiosperms, such as egg activation, zygote development and early embryogenesis, since the female gametophytes of plants are deeply embedded within ovaries. In this protocol, procedures for isolation of rice gametes, electro-fusion of gametes, and culture of the produced zygotes are described.
Keywords: Egg cellBackground
Fertilization and subsequent events in angiosperms, such as embryogenesis and endosperm development, occur in the embryo sac deeply embedded in ovular tissue (Nawaschin, 1898; Guignard, 1899; Russell, 1992; Raghavan, 2003). Therefore, isolated gametes have been used for in vitro fertilization (IVF) system to observe and analyze fertilization and postfertilization processes (reviewed in Wang et al., 2006). The IVF system used for angiosperms includes a combination of three basic micro-techniques: (i) the isolation and selection of male and female gametes; (ii) the fusion of pairs of gametes and (iii) single cell culture (Kranz, 1999). Procedures for isolating viable gametes have been established in a wide range of plant species, including monocotyledonous and dicotyledonous plants (reviewed in Kranz, 1999 and in Okamoto, 2011). The isolated gametes can be fused electrically (Kranz et al., 1991; Uchiumi et al., 2006 and 2007) or chemically using calcium (Faure et al., 1994; Kranz and Lörz, 1994; Khalequzzaman and Haq, 2005), polyethyleneglycol (Sun et al., 1995; Tian and Russell, 1997) or bovine serum albumin (Peng et al., 2005), as the gametes are generally protoplasts. Although gametes can be fused using these different procedures, only zygotes produced by electro-fusion are only known to divide and develop into embryo-like structures and plantlets. A complete IVF system was developed by Kranz and Lörz (1993) using maize gametes and electrical fusion, and, to take advantage of the abundant resources stemming from rice research, a rice IVF system was also established by Uchiumi et al. (2007). By the use of these electro-fusion based IVF systems, post-fertilization events, such as karyogamy (Faure et al., 1993; Ohnishi et al., 2014), egg activation and zygotic development (Kranz et al., 1995; Nakajima et al., 2010; Sato et al., 2010), paternal chromatin decondensation in zygote nucleus (Scholten et al., 2002), the microtubular architecture in egg cells and zygotes (Hoshino et al., 2004), fertilization-induced/suppressed gene expression (Okamoto et al., 2005), epigenetic resetting in early embryos (Jahnke and Scholten, 2009), have been successfully observed and investigated. Moreover, polyspermic triploid zygotes were produced by the modification of the rice IVF system and the triploid zygotes were grown into triploid mature plants (Toda et al., 2016). The rice IVF system described here might become an important technique for generating new cultivars with desirable characters as well as for investigating post fertilization events.
Materials and Reagents
- Coverslips (24 x 40 mm) (Thermo Fisher Scientific, Fisher Scientific, catalog number: 125485J ) (siliconized at the edges with 5% dichlorodimethylsilane in 1,1,1-trichloroethane, see Note 3)
- Glass capillaries made from 50 μl aspirator tubes (Drummond Scientific, catalog number: 2-000-050 ) (Figures 1A and 1B, Video 1; see Note 4)
- Manual handling injector (ST Science, type UJB)
- Non-treated plastic dishes with diameter of 3.5 cm (Iwaki, catalog number: 1000-035 )
- Razor blade
- Millicell-CM inserts, diameter 12 mm (EMD Millipore, catalog number: PICM01250 )
- Glass needles (2 mm diameter) with fine tips
- Rice plants (Oryza sativa L. cv. Nipponbare) (see Note 1)
- Feeder cells: rice suspension cell culture (Line Oc, provided by RIKEN Bio-Resource Center, Tsukuba, Japan) (see Note 2)
- Mineral oil (Sigma-Aldrich, catalog number: M8410-100ML )
- Mannitol (Wako Pure Chemicals Industries, catalog number: 133-00845 )
- Absolute ethanol (Wako Pure Chemicals Industries, catalog number: 057-00451 )
- 370 mosmol/kg H2O (330 mM) mannitol solution (autoclaved)
- 450 mosmol/kg H2O (385 mM) mannitol solution (autoclaved)
- 520 mosmol/kg H2O (430 mM) mannitol solution (autoclaved)
- CHU (N6) basal salt mixture (Sigma-Aldrich, catalog number: C1416 )
- Na2MoO4.2H2O (Wako Pure Chemicals Industries, catalog number: 514-30001 )
- CoCl2·6H2O (Wako Pure Chemicals Industries, catalog number: 036-03682 )
- CuSO4·5H2O (Wako Pure Chemicals Industries, catalog number: 039-19385 )
- Retinol (Sigma-Aldrich, catalog number: R7632 )
- Calciferol (Wako Pure Chemicals Industries, catalog number: 039-00291 )
- Biotin (Wako Pure Chemicals Industries, catalog number: 023-08711 )
- Thiamin·HCl (Nacalai Tesque, catalog number: 36319-82 )
- Nicotinic acid (Sigma-Aldrich, catalog number: N4126 )
- Pyridoxine·HCl (Wako Pure Chemicals Industries, catalog number: 163-05402 )
- Choline chloride (Sigma-Aldrich, catalog number: C7527 )
- Ca-pantothenate (Wako Pure Chemicals Industries, catalog number: 031-14161 )
- Riboflavin (Wako Pure Chemicals Industries, catalog number: 180-00171 )
- 2,4-D (Wako Pure Chemicals Industries, catalog number: 040-18532 )
- Cobalamine (Sigma-Aldrich, catalog number: V2876 )
- p-aminobenzoic acid (Sigma-Aldrich, catalog number: 100536 )
- Folic acid (Wako Pure Chemicals Industries, catalog number: 062-01801 )
- Ascorbic acid (Wako Pure Chemicals Industries, catalog number: 012-04802 )
- Malic acid (Sigma-Aldrich, catalog number: M1000 )
- Citric acid (Sigma-Aldrich, catalog number: 251275 )
- Fumaric acid (Wako Pure Chemicals Industries, catalog number: 069-00652 )
- Na-pyruvate (Tokyo Chemical Industry, catalog number: P0582 )
- Glutamine (Wako Pure Chemicals Industries, catalog number: 078-00525 )
- Casein hydrolysate (BD, catalog number: 22 3050 )
- Myo-inositol (Wako Pure Chemicals Industries, catalog number: 094-00281 )
- Glucose (Wako Pure Chemicals Industries, catalog number: 049-31165 )
- MS salt (Wako Pure Chemicals Industries, catalog number: 392-00591 )
- MS vitamin (Sigma-Aldrich, catalog number: M7150 )
- Sucrose (Wako Pure Chemicals Industries, catalog number: 196-00015 )
- Sorbitol (Wako Pure Chemicals Industries, catalog number: 198-03755 )
- 1-naphthaleneacetic acid (Nacalai Tesque, catalog number: 23628-32 )
- Kinetin (Sigma-Aldrich, catalog number: K3378 )
- Gelrite (Wako Pure Chemicals Industries, catalog number: 075-05655 )
- Dichlorodimethylsilane (Tokyo Chemical Industry, catalog number: D0358 )
- 1,1,1-trichloroethane (Tokyo Chemical Industry, catalog number: T0380 )
- Zygote culture medium (see Recipes)
- Regeneration media (see Recipes)
- Rooting media (see Recipes)
Equipment
- Stereo microscope (OLYMPUS, model: SZ61 )
- Inverted microscope (OLYMPUS, model: IX-71 or IX-73 )
- Forceps
- Electrofusion apparatus (Nepa Gene, model: ECFG21 )
- Manipulator (NARISHIGE, model: M-152 ) with a double pipette holder (NARISHIGE, model: HD-21 )
- Electrodes (Nepa Gene, model: CUY5100Ti100 ) fixed to the pipette holder
- Sliding stage for the insertion of a coverslip and a plastic dish
- Environmental chambers
- Charge-coupled device camera (Pixcera, model: Penguin 600CL )
Software
- InStudio software (Pixcera)
Procedure
- Isolation of gametes
- Overlay the siliconized coverslip with 0.3 ml mineral oil and make several 1-2 μl mannitol droplets (370 mosmol/kg H2O) inside the mineral oil on the coverslip (Figure 1C) using a glass capillary connected to a handling injector. Take care that the droplets have no access to the air (Figure 1D).
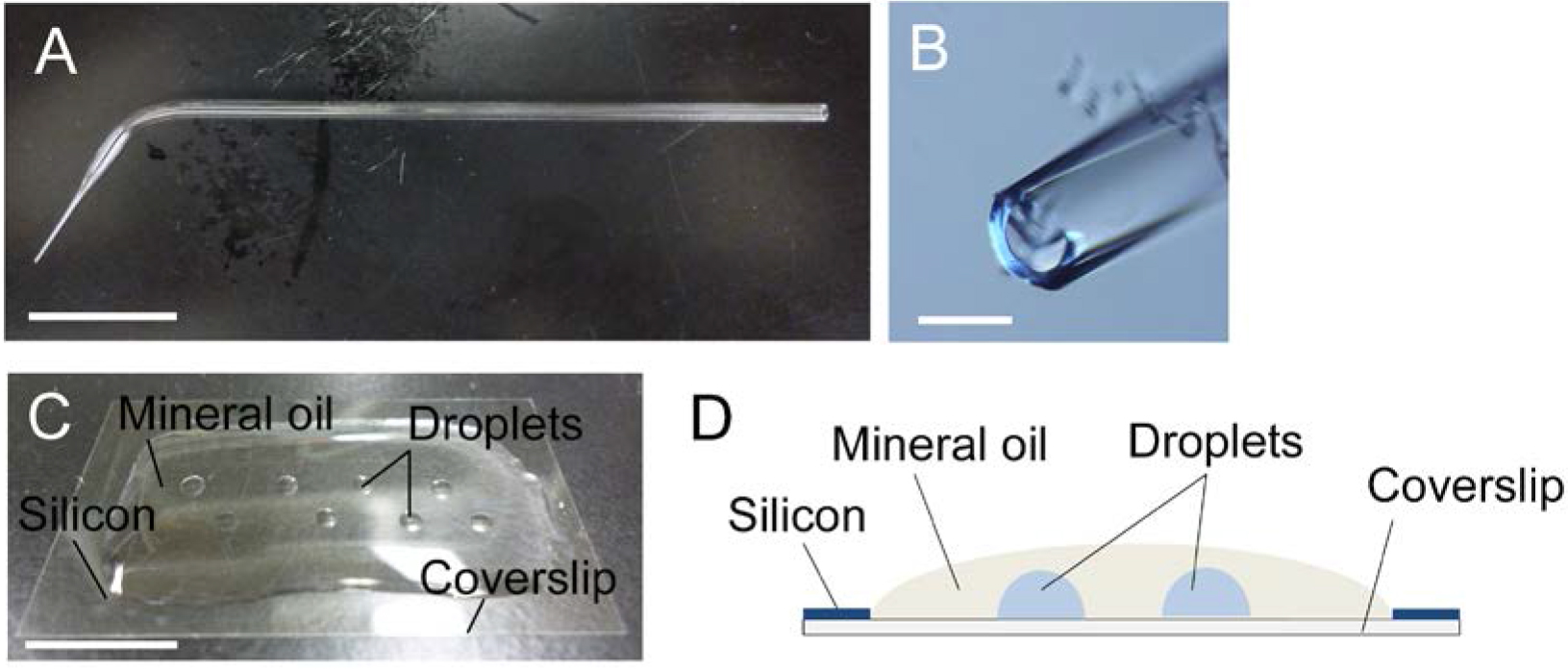
Figure 1. Glass capillary (A, B) and mannitol droplets on coverslip (C, D). A. A glass capillary drawn by hand. B. Tip opening of the glass capillary in panel A. C and D. A photo (C) and an image (D) of mannitol droplets inside the mineral oil over the siliconized coverslip. Bars = 1 cm in A and C, 200 μm in B.Video 1. Making glass capillary - Collect panicles in which some flowers have already opened and others remain unflowered. Pick up the unflowered ones from the panicles and dissect them under a stereo microscope. Isolate ovaries, and transfer them into 3.5 cm plastic dishes filled with 3 ml of mannitol solution (370 mosmol/kg H2O) (Figure 2A).
- For egg cell isolation, remove the stigmas from ovaries and transfer them into new 3.5 cm plastic dishes filled with 3 ml of the mannitol solution (see Note 5). Allow the ovaries to sink to the bottom of the dishes and cut them transversely with a razor blade at the middle (Figures 2A and 2B). Under an inverted microscope, identify the egg cells which are released from the lower parts of the cut ovaries (Figure 2C; see Note 6). By using a glass capillary connected to a handling injector, collect egg cells and transfer them into mannitol droplets prepared in step A1 (Figure 4A; see Note 7).
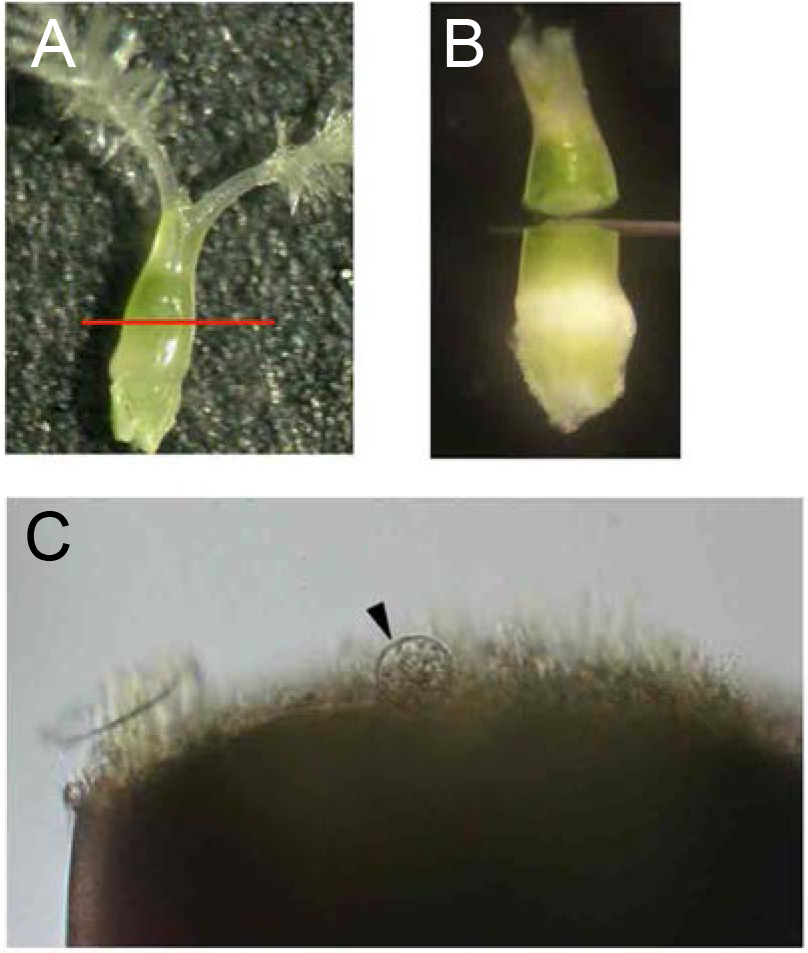
Figure 2. Isolation of rice egg cells from ovaries. A. Ovary harvested from a rice flower before flowering. The red line indicates the incision line for egg isolation. B. Cut ovary in mannitol solution. C. Rice egg cell (arrowhead) being released from basal portion of the dissected ovary. - For sperm cell isolation, dissect an unopened flower under a stereo microscope to obtain anthers, and transfer anthers into a 3.5 cm plastic dish filled with 3 ml of mannitol solution (370 mosmol/kg H2O). Break anthers in mannitol solution with forceps to free pollen grains (Figure 3A). Pollen grains burst due to the osmotic shock and release sperm cells. Identify the sperm cells under an inverted microscope (Figure 3B). By using a glass capillary connected to a handling injector, collect sperm cells and transfer into mannitol droplets prepared in step A1. (Figure 4B; see Note 8).
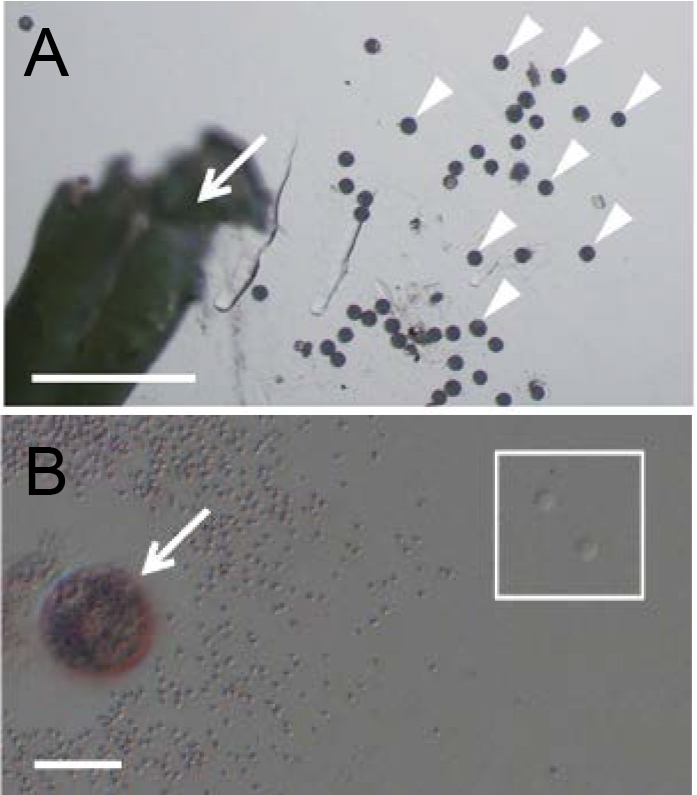
Figure 3. Isolation of rice sperm cells from pollen grains. A. A broken anther (arrow) and released pollen grains (arrowheads) in mannitol solution. B. A pollen grain (arrow) released its content in mannitol solution. Two sperm cells were enclosed with the square. Bars = 500 µm in A and 20 μm in B.
- Overlay the siliconized coverslip with 0.3 ml mineral oil and make several 1-2 μl mannitol droplets (370 mosmol/kg H2O) inside the mineral oil on the coverslip (Figure 1C) using a glass capillary connected to a handling injector. Take care that the droplets have no access to the air (Figure 1D).
- Electro-fusion of isolated gametes
- Set up fusion apparatus and adjust the position of electrodes (Figures 4C and 5).
- Transfer one egg cell to each of the six mannitol droplets (Figure 4C), then transfer 1 or 2 sperm cells to each droplet.
- Lower the position of electrodes into a fusion droplet using manipulator (Videos 2 and 3), and align and fix the two gametes at one electrode under an alternating current (AC) field (1 MHz, 5 V rms). Under the AC field, gametes move toward electrode. By moving the electrode connected with manipulator, first fix an egg cell to the electrode. Using the same procedure, fix a sperm cell to the female gamete (Figure 4D). Adjust the final distance of the electrodes to approximately twice the sum of the diameters of the cells.
- Add 0.5-1.0 μl of mannitol solution (520 mosmol/kg H2O) gently to the fusion droplet using a thin glass capillary (Figure 4E, see Note 9).
- Induce cell fusion by applying a single negative direct current (DC) pulse (50 μsec, 12-15 V) (Figures 4F and 4G, Video 4, see Note 10).
- Remove the fusion products from the electrode by gently moving the electrode. Move the electrodes out of the droplet with manipulator and conduct the next gamete fusion at step B3 (see Notes 11 and 12).
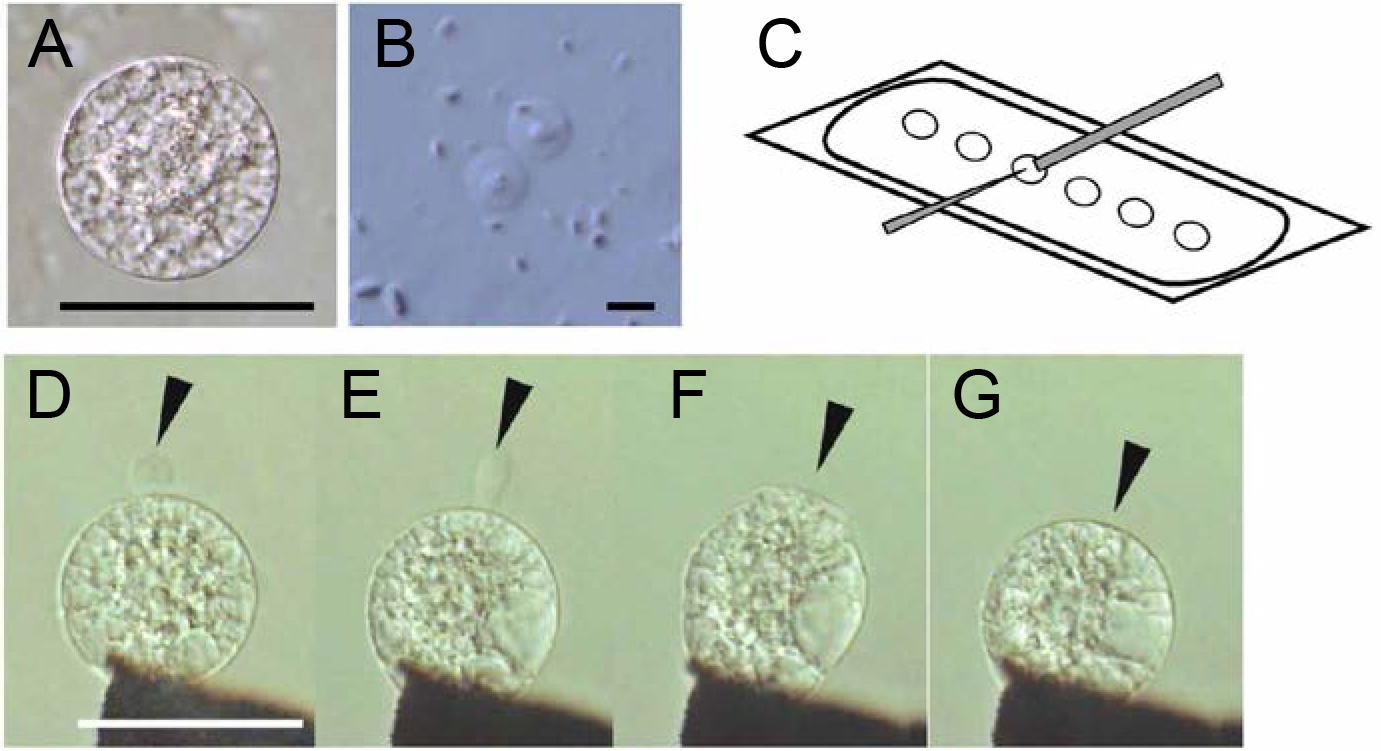
Figure 4. Isolated rice gametes (A and B) and electro-fusion of gametes (C–G). A. An isolated rice egg cell. B. Rice sperm cells released from pollen grain. C. An illustration of the fusion droplets on a coverslip covered with mineral oil. The gray bar and thin triangle indicate electrodes. D. Alignment of an egg cell with a sperm cell (arrowhead) on one of the electrodes under an alternating current (AC) field in a fusion droplet. E. Aligned egg and sperm cells after the addition of mannitol solution with a higher osmolality to the fusion drop. The sperm cell becomes oblong (arrowhead). F. Fusion of gametes following a negative direct current (DC) pulse. An arrowhead indicates fusion point. G. A zygote 10 sec after fusion. The arrowhead indicates the fusion point. Bars = 50 μm in A, D and 5 μm in B.
Figure 5. Fusion system consisting of inverted microscope, electrofusion apparatus, manipulator and electrodesVideo 2. Adjustment of electrodes into mannitol droplet in which an egg cell and a sperm cell have been transferredVideo 3. Electrodes in mannitol dropletVideo 4. Electrofusion of rice gametes
- Set up fusion apparatus and adjust the position of electrodes (Figures 4C and 5).
- Culture and development of zygotes
- Place 0.2 ml zygote culture medium in a Millicell-CM insert and put it into a 3.5 cm plastic dish containing 2 ml of the medium. Add 40-60 µl of a rice suspension cell culture into the dish as feeder cells.
- After sterilization of the microcapillary by washing with absolute ethanol and sterilized water, transfer IVF-produced zygotes into fresh mannitol droplets (450 mosmol/kg H2O) twice and then transfer them onto the membranes of a Millicell-CM insert (Figure 5A).
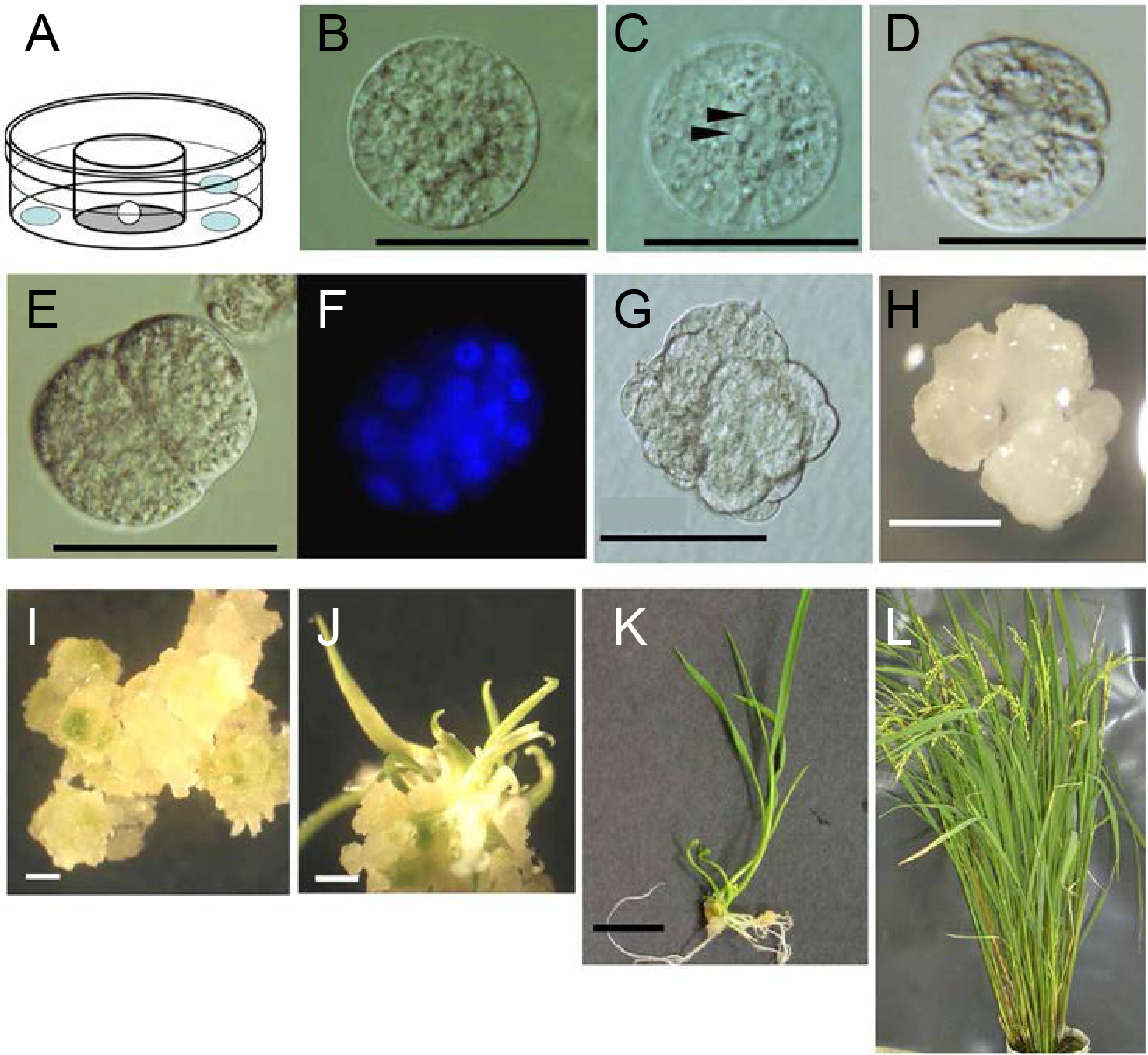
Figure 6. Culture and development of zygotes. A. An illustration of zygote culture; A white circle in the Millicell insert indicates a zygote. Light-blue oblong circles represent aggregates of feeder cells. B. A zygote 1 h after fusion; C. A zygote 4 h after fusion; Two nucleoli are indicated by arrowheads. D. An asymmetric two-celled embryo 18 h after fusion; E and F. Nuclear staining of an embryo 48 h after fusion, visualized by brightfield and fluorescence microscopy, respectively; G. A cell mass 5 days after fusion, which developed from the globular-like embryo; H. A white cell colony 18 days after fusion; I. A developed cell colony 4 days after transferring the white cell colony (panel H) into regeneration medium (22 days after fusion). Green spots are visible in/on the cell colony. J. Regenerated shoots; Generation of shoots can be observed after 8 days of subculturing the white cell colony (26 days after fusion). K. A plantlet after 12 days of subculturing a regenerated shoot in hormone-free medium (43 days after fusion); L. A regenerated plant with seed sets (100 days after fusion). Bars = 50 μm in B-E and G, 1 mm in H-J and 1 cm in K. - After overnight culture of zygotes at 26 °C in the dark without shaking, continue culture with gentle shaking (40 rpm) (Figures 6B-6F; see Notes 13 and 14).
- Five days after fusion, remove feeder cells by transferring the Millicell dishes containing the embryos into new 35-mm diameter dishes filled with 2 ml of fresh zygote culture medium (Figure 6G; see Note 15). Continue culturing as above.
- After 18 days in culture, subculture cell colonies developed from the IVF-produced zygotes in Millicell-CM inserts onto a regeneration medium in plastic dishes by use of a sterilized Pasteur pipette (Note 16). Incubate under continuous light at 30 °C for 12-30 days (Figure 6H; see Note 17).
- Transfer the differentiated shoots into a rooting medium in plastic dishes and culture them under a 13/11 h light/dark cycle at 28 °C for 11-13 days (Figures 6I and 6J).
- Transfer the resulting plantlets to soil pods (diameter 20-28 cm, depth 30-35 cm) and grow in environmental chambers as described in Note 1 (Figure 6K). If needed, harvest seeds from the regenerated plants and germinate them (Figure 6L).
- Place 0.2 ml zygote culture medium in a Millicell-CM insert and put it into a 3.5 cm plastic dish containing 2 ml of the medium. Add 40-60 µl of a rice suspension cell culture into the dish as feeder cells.
Data analysis
Digital images of gametes, zygotes, and their resulting embryos were obtained using a cooled charge-coupled device camera (Penguin 600CL; Pixcera, Los Gatos, CA, USA) and InStudio software (Pixcera).
Notes
- Rice plants are grown in environmental chamber at 26 °C in a 13/11 h light/dark cycle with a photosynthetic photon flux density of 150-300 μmol photons m-2 sec-1. Under these growth conditions, flowers can be obtained throughout all seasons.
- Rice suspension cell, Line Oc, were subcultured once weekly according to instructions from RIKEN Bio-Resource Center. No difference in feeder effects between freshly subcultured cells and one week-cultured cells has been observed.
- Coverslips should be non-coated, as using coated coverslips will result in attachment of the cells to the surface of the coverslip.
- Tip openings were drawn by hand to 150-250 μm.
- Without removing the stigmas, ovaries always float on the mannitol solution. To isolate egg cells, sinking ovaries into the mannitol solution is essential. Usually, 15-25 ovaries are put into a dish.
- Usually, 3-8 egg cells are automatically released from approximately 20 cut ovaries. Gentle pushing of the basal portion of the lower part of the cut ovary with a glass needle will produce additional egg cells.
- Egg cells can be kept in the mannitol droplet until 6 h after isolation to conduct IVF without decreasing fusion efficiency. Alternatively, egg cells can be stored at 4 °C overnight for next day use.
- Sperm cells should be used for IVF within 1 h after isolation. Otherwise, sperm cells appear to degenerate and cannot be fused with egg cells.
- The addition of mannitol solution with a higher osmolarity changes the shape of the sperm cell to oblong and makes the attachment of the egg cell to the electrode more stable (Figures 4D and 4E). Without this treatment, egg cells are often released from the electrode upon fusion induced by a DC pulse and fusion efficiency is greatly reduced.
- If no cell fusion occurs, reduce the distance between the two electrodes and pulse again.
- At each round of fusion procedures, 5-6 sets of gamete fusions are recommended (Figure 4C), since conducting many sets of gamete fusion takes time and sperm cell will be degenerate during the course of the experiments.
- The efficiency of successful electrofusion is approximately 85% under optimal conditions. 20 to 40 egg cells can be isolated from one hundred processed ovaries, and 15-30 egg cells can be fused with sperm cells by one experimenter in a day.
- The rice zygotes produced by IVF start to form cell walls (Figure 6B) and two nucleoli can be observed in a zygote at least 4 h after fusion (Figure 6C). At around 12 h after fusion, well-developed granular organelles, probably starch granules, are visible in the zygotes and the first asymmetric cell division of the zygotes is observed at 17-22 h after fusion (Figure 6D). After the first division, the two-celled embryos continue to develop into early embryos at 40-50 h after fusion (Figures 6E and 6F).
- Approximately 90% IVF-produced zygotes divide into two-celled embryo, and 90% IVF-produced two-celled embryos develop into globular embryos.
- After five days culture of the IVF-produced zygotes, co-cultivation with feeder cells is not needed.
- Diameter of cell colonies are approximate 1-2 mm, and can be transferred with Pasteur pipette.
- Normally, after four days of subculture of the cell colony on a solidified-regeneration medium (22 days after fusion), green spots become visible and the emergence of multiple shoots is observed after eight days of subculturing (26 days after fusion). Most subcultured cell colonies form green spots and multiple shoots.
Recipes
- Zygote culture medium (N6Z-medium [Kumlehn et al., 1998] with modifications)
2 g/L CHU (N6) basal salt mixture
0.025 mg/L Na2MoO4·2H2O
0.025 mg/L CoCl2·6H2O
0.025 mg/L CuSO4·5H2O
0.01 mg/L retinol
0.01 mg/L calciferol
0.01 mg/L biotin
1 mg/L thiamin·HCl
1 mg/L nicotinic acid
1 mg/L pyridoxine·HCl
1 mg/L cholin chloride
1 mg/L Ca-pantothene
0.2 mg/L riboflavin
0.2 mg/L 2,4-D
0.02 mg/L cobalamine
0.02 mg/L p-aminobenzoic acid
0.4 mg/L folic acid
2 mg/L ascorbic acid
40 mg/L malic acid
40 mg/L citric acid
40 mg/L fumaric acid
20 mg/L Na-pyruvate
1000 mg/L glutamine
250 mg/L casein hydrolysate
100 mg/L myo-inositol
Osmolality, 450 mosmol kg-1 H2O adjusted with glucose
Adjust to pH 5.7 and filter sterilized - Regeneration media (Solidified MS medium with some modifications [Hiei et al., 1994])
MS salt
MS vitamin
100 mg/L myo-inositol
2 g/L casamino acid
30 g/L sucrose
30 g/L sorbitol
0.2 mg/L 1-naphthaleneacetic acid (NAA)
1 mg/L kinetin
0.3% gelrite - Rooting media
The same as the regeneration media but omitting sorbitol, kinetin and NAA
Acknowledgments
This protocol was adapted from Uchiumi et al. (2007) and Okamoto (2010). This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid No. 26113715 to T.O.) and the Japan Society for the Promotion of Science (Grant-in-Aid No. 16K14742 to T.O.). I thank Ms. Tomoko Mochizuki (Tokyo Metropolitan University) for technical assistance.
References
- Faure, J. E., Digonnet, C. and Dumas, C. (1994). An in vitro system for adhesion and fusion of maize gametes. Science 263(5153): 1598-1600.
- Faure, J. E., Mogensen, H. L., Dumas, C., Lorz, H. and Kranz, E. (1993). Karyogamy after electrofusion of single egg and sperm cell protoplasts from maize: cytological evidence and time course. Plant Cell 5(7): 747-755.
- Guignard, M. L. (1899). Sur les antherozoides et la double copulation sexuelle chez les vegetaux angiosperms. Rev Gén Bot 11: 129-135.
- Jahnke, S. and Scholten, S. (2009). Epigenetic resetting of a gene imprinted in plant embryos. Curr Biol 19(19): 1677-1681.
- Hiei, Y., Ohta, S., Komari, T. and Kumashiro, T. (1994). Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6(2): 271-282.
- Hoshino, Y., Scholten, S., von Wiegen, P., Lörz, H. and Kranz, E. (2004). Fertilization induced changes in the microtubular architecture of the maize egg cell and zygote - an immunocytochemical approach adapted to single cells. Sex Plant Reprod 17(2): 89-95.
- Khalequzzaman, M. and Haq, N. (2005). Isolation and in vitro fusion of egg and sperm cells in Oryza sativa. Plant Physiol Biochem 43(1): 69-75.
- Kranz, E. (1999). In vitro fertilization with isolated single gametes. Methods Mol Biol 111: 259-267.
- Kranz, E., Bautor, J. and Lörz, H. (1991). In vitro fertilization of single, isolated gametes of maize mediated by electrofusion. Sex Plant Reprod 4(1): 12-16.
- Kranz, E. and Lörz, H. (1993). In vitro fertilization with isolated, single gametes results in zygotic embryogenesis and fertile maize plants. Plant Cell 5(7): 739-746.
- Kranz, E. and Lörz, H. (1994). In vitro fertilization of maize by single egg and sperm cell protoplast fusion mediated by high calcium and high pH. Zygote 2(2): 125-128.
- Kranz, E., Wiegen, P. and Lörz, H. (1995). Early cytological events after induction of cell division in egg cells and zygote development following in vitro fertilization with angiosperm gametes. Plant J 8(1): 9-23.
- Kumlehn, J., Lörz, H. and Kranz, E. (1998). Differentiation of isolated wheat zygotes into embryos and normal plants. Planta 205(3): 327-333.
- Nakajima, K., Uchiumi, T. and Okamoto, T. (2010). Positional relationship between the gamete fusion site and the first division plane in the rice zygote. J Exp Bot 61(11): 3101-3105.
- Nawaschin, S. (1989). Resultate einer Revision der Befruchtungsvorgänge bei Lilium Martagon und Fritillaria tenella. Bulletin de l'Académie Impériale des Sciences 9(4):377-382.
- Ohnishi, Y., Hoshino, R. and Okamoto, T. (2014). Dynamics of male and female chromatin during karyogamy in rice zygotes. Plant Physiol 165(4): 1533-1543.
- Okamoto, T. (2011). In vitro fertilization with isolated rice gametes: production of zygotes and zygote and embryo culture. Methods Mol Biol 710:17-27.
- Okamoto, T., Scholten, S., Lörz, H. and Kranz, E. (2005). Identification of genes that are up-or down-regulated in the apical or basal cell of maize two-celled embryos and monitoring their expression during zygote development by a cell manipulation-and PCR-based approach. Plant Cell Physiol 46(2): 332-338.
- Peng, X. B., Sun, M. X. and Yang, H. Y. (2005). A novel in vitro system for gamete fusion in maize. Cell Res 15(9): 734-738.
- Raghavan, V. (2003). Some reflections on double fertilization, from its discovery to the present. New Phytologist 159(3): 565-583.
- Russell, S. D. (1992). Double fertilization. Int Rev Cytol 140: 357-388.
- Sato, A., Toyooka, K. and Okamoto, T. (2010). Asymmetric cell division of rice zygotes located in embryo sac and produced by in vitro fertilization. Sex Plant Reprod 23(3): 211-217.
- Scholten, S., Lorz, H. and Kranz, E. (2002). Paternal mRNA and protein synthesis coincides with male chromatin decondensation in maize zygotes. Plant J 32(2): 221-231.
- Sun, M., Yang, H. and Zhou, C. (1995). Single-pair fusion of various combinations between female gametoplasts and other protoplasts in Nicotiana tabacum. Acta Bot Sin 37: 1-6.
- Tian, H. Q. and Russell, S. D. (1997). Micromanipulation of male and female gametes of Nicotiana tabacum: II. Preliminary attempts for in vitro fertilization and egg cell culture. Plant cell reports 16(9): 657-661.
- Toda, E., Ohnishi, Y. and Okamoto, T. (2016). Development of polyspermic rice zygotes. Plant Physiol 171(1): 206-214.
- Uchiumi, T., Komatsu S., Koshiba, T. and Okamoto, T. (2006). Isolation of gametes and central cells from Oryza sativa L. Sex Plant Reprod 19(1): 37-45.
- Uchiumi, T., Uemura, I. and Okamoto, T. (2007). Establishment of an in vitro fertilization system in rice (Oryza sativa L.). Planta 226(3): 581-589.
- Wang, Y. Y., Kuang, A., Russell, S. D. and Tian, H. Q. (2006). In vitro fertilization as a tool for investigating sexual reproduction of angiosperms. Sexual Plant Reproduction 19(3): 103-115.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Toda, E., Ohnishi, Y. and Okamoto, T. (2016). Electro-fusion of Gametes and Subsequent Culture of Zygotes in Rice. Bio-protocol 6(24): e2074. DOI: 10.21769/BioProtoc.2074.
-
Toda, E., Ohnishi, Y. and Okamoto, T. (2016). Development of polyspermic rice zygotes. Plant Physiol 171(1): 206-214.
Category
Plant Science > Plant developmental biology > General
Cell Biology > Cell engineering > Cell fusion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










