- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Aorta Ring Assay
Published: Vol 6, Iss 13, Jul 5, 2016 DOI: 10.21769/BioProtoc.1856 Views: 15456
Reviewed by: Letizia De ChiaraAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Improved Protocol for the Matrigel Duplex Assay: A Method to Measure Retinal Angiogenesis
Kathleen C. Brown [...] Piyali Dasgupta
Dec 5, 2023 1905 Views

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3758 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2626 Views
Abstract
Angiogenesis is the nature and pathological process of blood vessel growth from pre-existing vascular buds. It plays an important role in cancer and cardiovascular disease. The aorta ring assay is an approach to study angiogenesis. In this experiment, we used the aorta of rat as the study material, cleaned the surrounding tissue of aorta and cut it into 1 mm long rings. Next, the rings were cultured in growth factor-reduced matrigel polymerized at 37 °C. Angiogenesis was assessed at 7 days by using an inverted microscope platform.
Materials and Reagents
- 10 cm dish (Thermo Scientific, catalog number: 172931 )
- 24 well plates (Thermo Scientific, catalog number: 142475 )
- 2 ml and 5 ml disposable sterilized syringe
- Sprague-Dawley 4-week old rat (Zhejiang Chinese Medical University Animal Center)
- Growth factor-reduced matrigel (Corning, catalog number: 354230 )
- Dulbecco’s Modification of Eagle’s Medium with 1 g/L glucose glutamine & sodium pyruvate (Mediatech, catalog number: 10-014-CV )
- Fetal Bovine Serum (Biological Industries, catalog number: 04-001-1A )
- Phosphate buffer saline (PBS) (Shanghai ji’nuo, catalog number: GNM 20012 )
- Chloral hydrate (Guoyao chemical reagent co. LTD, catalog number: 30037517 )
- 70% ethanol
- Medium (see Recipes)
- 4% chloral hydrate (see Recipes)
Equipment
- Surgical scissors, scalpel and tweezers
- Ruler
- 37 °C, 5% CO2 cell culture incubator (Thermo Fisher Scientific, catalog number: 51026334 )
- Inverted microscope (Leica Microsystems, model: DMi1 )
- Electronic scale
Software
- Image-Pro Plus 6.0
Procedure
Note: The whole experiment should be in aseptic conditions including all materials and reagents. All operations should be sterile.
- Allow the growth factor-reduced matrigel melt overnight from -20 °C to 4 °C in a refrigerator.
- Put 24 well plates and pipette tips in -20 °C chilled overnight.
- Use electronic scale to weigh rat.
- Use 5 ml disposable sterilized syringe to inject 4% chloral hydrate to rat’s abdomen, 1 ml chloral hydrate per 100 g to anesthesia rat then sacrifice the animal through cervical dislocation, place the rat on the animal operation asepsis.
- Spray 70% ethanol to rat’s skin and bundle the rat.
- Use scissors to open the chest and remove other organs to expose the Thoracic aorta (Figures 1 and 2).
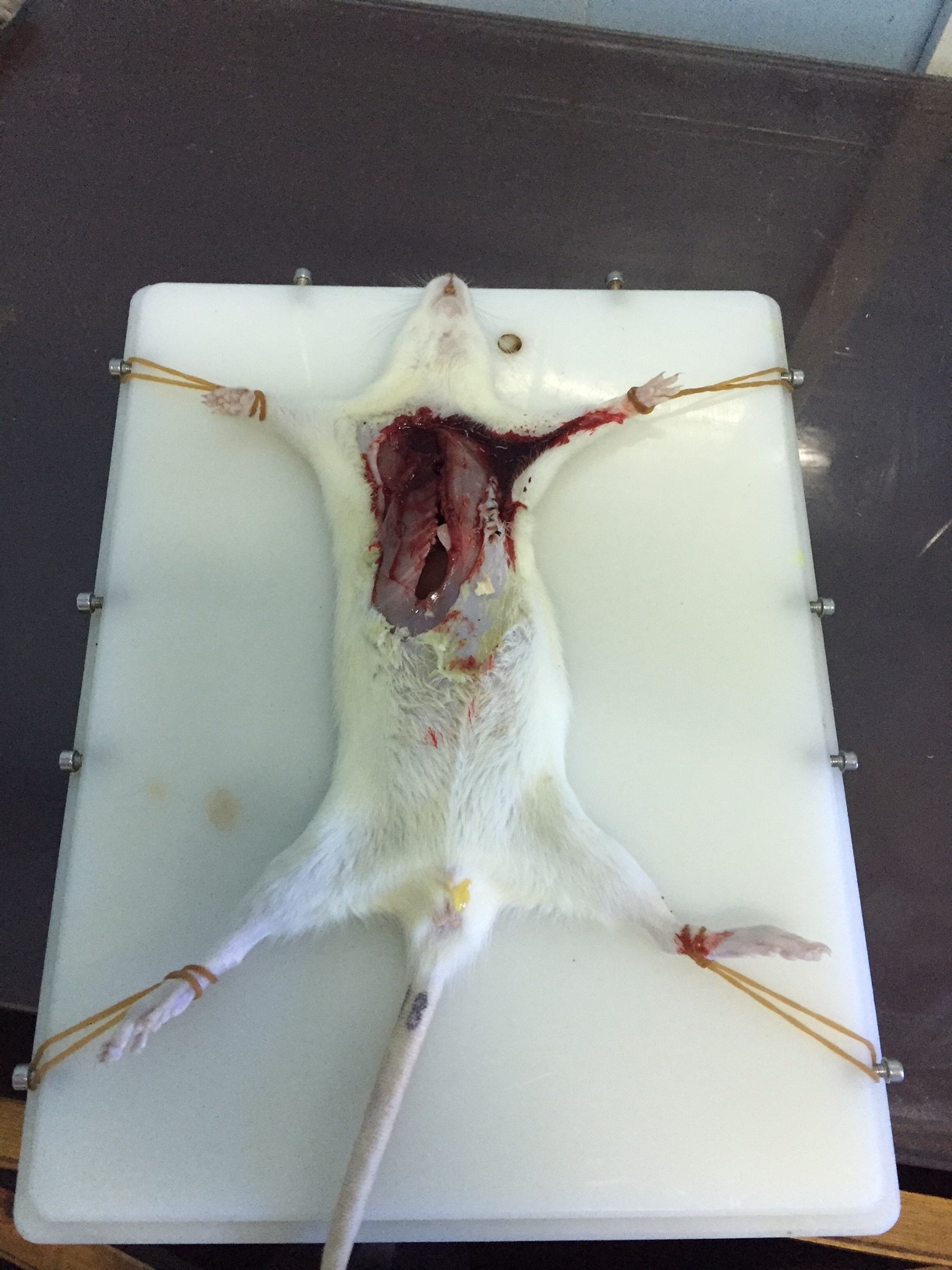
Figure 1. Place the rat on the animal operation asepsis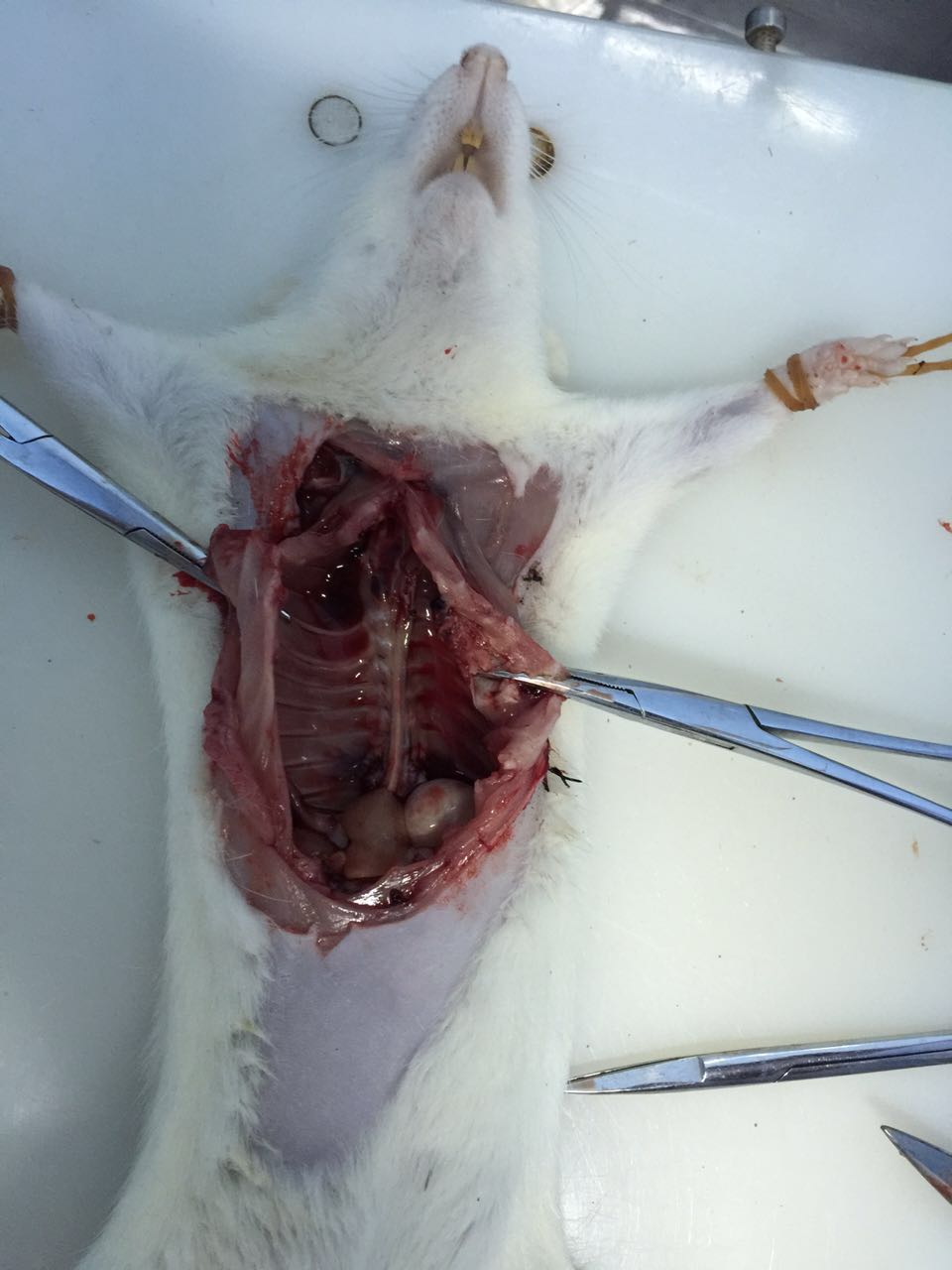
Figure 2. Open the chest and remove other organs to expose the thoracic aorta
- Use tweezers and scissors to separate the spine and aorta (Figure 3).
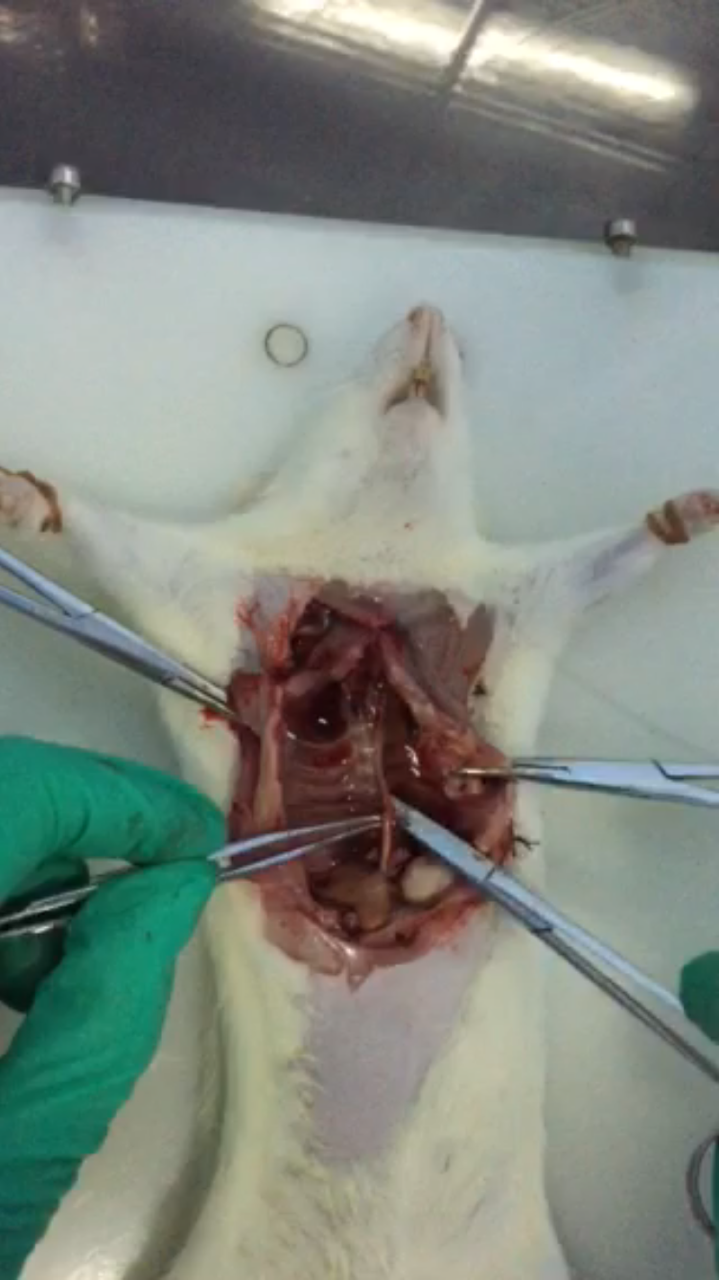
Figure 3. Use tweezers and scissors to separate spine and aorta
- Cut the aorta from diaphragm to the end of the heart, then put it in 10 cm dish and wash the aorta with sterile PBS at room temperature (Figure 4).
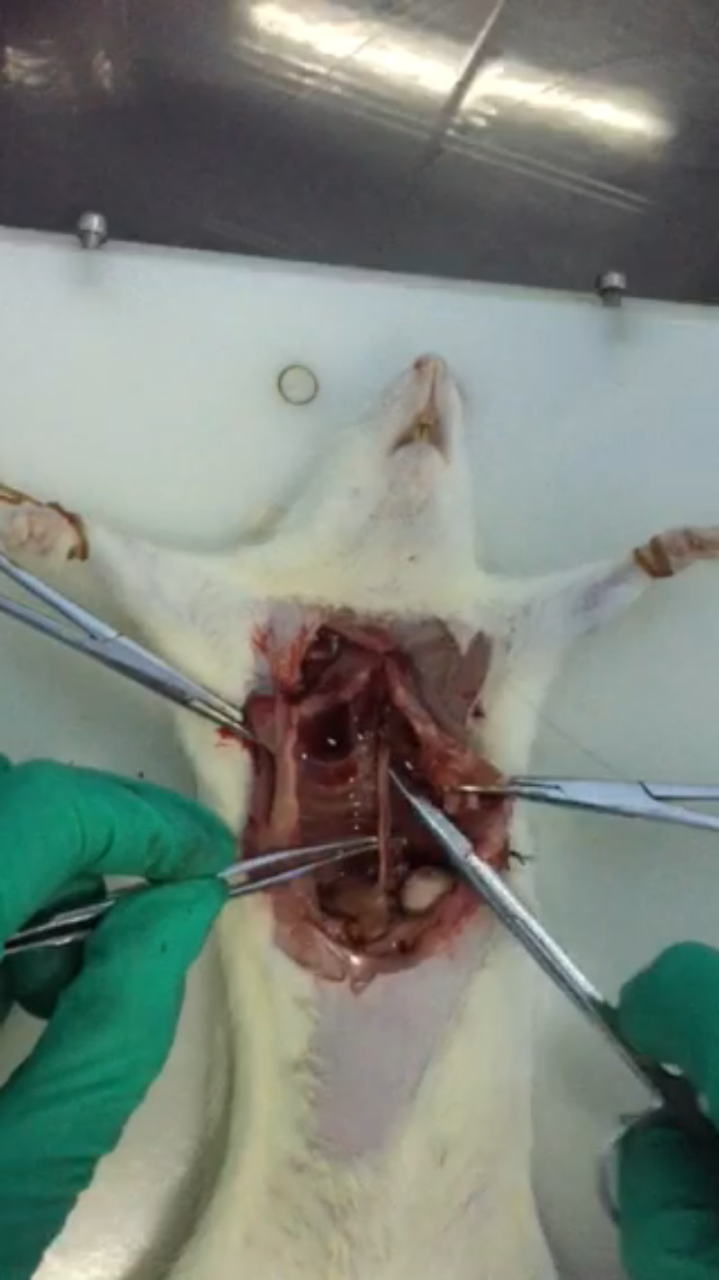
Figure 4. Cut aorta from diaphragm to the end of the heart
- Carefully clean the surrounding tissue around theaorta (Figure 5).

Figure 5. Clean the surrounding tissue around the aorta
- Use scalpel to cut the aorta into sections as 1 mm long rings measured by a ruler (Figure 6).
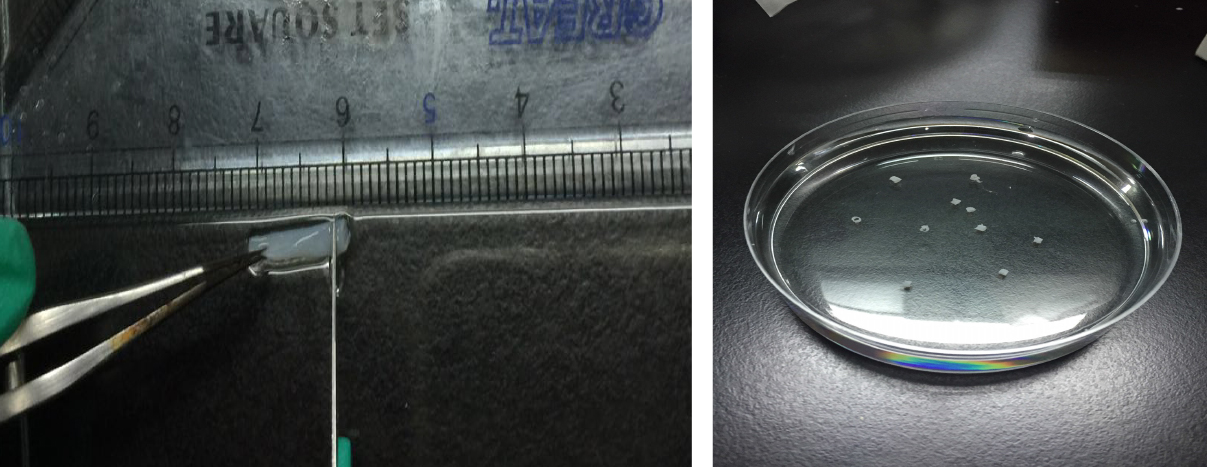
Figure 6. Use scalpel to cut aorta into sections as 1 mm long rings measured by a ruler
- Keep the growth factor-reduced matrigel on ice. Then add 150 µl matrigel into each well in 24 well plates in biosafety cabinet. Three complex wells for each group.
Notes:- The matrigel should be careful and quick. It’s easy to become solidified at room temperature.
- Keep the matrigel on ice before adding it to the well, but the plate does not need to be put on ice, the plate should be chilled before the matrigel is added.
- After adding the matrigel gently shake the plate to be sure the whole surface is covered by matrigel. If there are some bubbles in the well use 2 ml sterilized syringe to break them.
- Gently shake the plate then leave it in an ordinary humidified incubator for 30 min (37 °C).
- Put the 1 mm aorta ring in the middle of each well.
- Then incubate for about 10 min in the ordinary humidified incubator (37 °C).
- Add another 150 µl matrigel into the well to cover the ring.
- Then incubate for 30 min again in the ordinary humidified incubator (37 °C).
- As the aorta ring is embedded in the matrigel then add 200 µl the Dulbecco’s Modification of Eagle’s Medium with 10% Fetal Bovine Serum per well, finally put 24 well plates in the incubator (37 °C) (Figure 7)

Figure 7. The aorta ring in the matrigel covered by medium in the 24 well plates - Refresh the medium every 2 d.
- After 7 d, the branch of vascular is observed under microscope (Figure 8).

Figure 8. After 7 days, the branch of vascular is observed by microscope. Scale bar, 100 µm. - Use Image-Pro Plus 6.0 to detect the sprouting length and range.
Total vessel outgrowth is summed for each ring using Image-Pro Plus 6.0 software. The total length of all branches of each ring is calculated so that we can compare them within groups.
Notes
- In this protocol we only use one kind of medium (a common medium) to culture.
- The students can also collect different kinds of condition media to make more than one group. Usually we set up several groups to compare different supernatants working on the aorta ring.
- When you have several groups you need to set up three complex wells for each group.
Recipes
- Medium
90% Dulbecco’s Modification of Eagle’s Medium
10% Fetal Bovine Serum - 4% chloral hydrate
0.4 g chloral hydrate
10 ml deionized water
Acknowledgments
We thank Han et al. for technical advice on Aorta Ring Assay method previously published in Angiogenesis 2012. This work was supported by grants from National Natural Science Foundation of China (No.31171418,81320108003,31371498 for J.W., No.81170308,81370247 for X.Y.H., No.81202948 for L.Z., No.81100141 for J.J.), National High-tech R&D 863 Program (No.2013AA020101) Science and Technology Department of Zhejiang province public welfare projects (No.2013C37054), The National Basic Research Program of China (973Program, No.2014CB965100, 2014CB965103), Major science and technology projects of Zhejiang province (2012C13013-3), National Natural Science Foundation of Chian (No.81573641 for LZ), Zhejiang Provincial Natural Science Foundation (No.LY16H280003 for LZ).
References
- Han, Y., Yang, K., Proweller, A., Zhou, G., Jain, M. K. and Ramirez-Bergeron, D. L. (2012). Inhibition of ARNT severely compromises endothelial cell viability and function in response to moderate hypoxia. Angiogenesis 15(3): 409-420.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jin, J., Hu, X., Zhang, L. and Wang, J. (2016). Aorta Ring Assay. Bio-protocol 6(13): e1856. DOI: 10.21769/BioProtoc.1856.
Category
Developmental Biology > Cell growth and fate > Angiogenesis
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link