- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Sucrose Preference Test to Measure Stress-induced Anhedonia
Published: Vol 6, Iss 11, Jun 5, 2016 DOI: 10.21769/BioProtoc.1822 Views: 33488
Reviewed by: Soyun KimAdler R. DillmanXi Feng

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1294 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1470 Views
Abstract
The 2-bottle choice procedure for assessing sucrose preference is a useful test to investigate anhedonia (i.e., inability to feel pleasure) in laboratory rodents, particularly in stress-based models of depression. The 2-bottle choice procedure allows for a comparison between behavioral preference for sucrose solution in drinking water compared to water only. Preference is measured by volume and/or weight of liquid consumed daily, which is then converted to a percent preference compared to a water only baseline period. Sucrose preference is attenuated by a diversity of chronic stressors, including chronic mild and unpredictable stress (Willner et al., 1992; Willner, 1997; Pothion et al., 2004) and social defeat stress (Krishnan et al., 2007). It may also be susceptible to perturbation in mouse models of drug addiction because sucrose preference is altered in drug-dependent individuals (Kampov-Polevoy et al., 1997; Bogucka-Bonikowska et al., 2002; Janowsky et al., 2003). Both stress- and drug-induced alterations in sucrose preference may stem from maladaptations in the reward pathway, which consists of the dopaminergic neurons extending from the ventral tegmental area to the nucleus accumbens (NAc). Indeed, alterations in cyclic-AMP response element binding protein (CREB) activity in NAc underlie preference for sucrose (Barrot et al., 2002). Additionally, the transcription factor ΔFosB in NAc (Wallace et al., 2008), but not dorsal hippocampus (Eagle et al., 2015), regulates natural rewards, such as sucrose consumption. Therefore, the sucrose preference test described below provides a well-validated model to assess anhedonia and the function of specific brain regions and circuits.
Keywords: AnhedoniaMaterials and Reagents
- Sipper caps, composed of 2.5 cm straight stainless steel sipper tubes (VWR International, catalog number: 10718-330 ) inserted into rubber stoppers (VWR International, catalog number: 59581-287 )
- 50 ml conical centrifuge tubes (VWR International, catalog number: 89039-656 )
- Adult (7 weeks or older) mice (C57BL/6J) (The Jackson Laboratory)
Note: Alternate strains and ages of mice may also be used. Mice are housed singly, in an environment with controlled temperature (around 23 °C) and humidity under a 12-12 h light-dark cycle with food and water ad libitum. See Animal considerations in Notes for more details. - Sucrose (crystals)
- Reverse osmosis (RO) filtered water
Note: Alternatively substitute with the mouse’s normal drinking water.
Equipment
- Mouse caging
- Polycarbonate tubs (Mouse Cage; 7 ½ in W x 11 ½ in L x 5 in H) (Ancare) with standard woodchip mouse bedding (NORTHEASTERN PRODUCTS CORP., Aspen Chip)
Note: Standard cage changes are allowed during the acclimation period (see below), however it is recommended to avoid cage changes during the period of data collection (Procedure steps 2-5) to prevent leakage. Leakage should be avoided to reduce undesired variability. - Stainless steel wire cage lid (Ancare, model: N10SSR Mouse Lid )
- Polycarbonate tubs (Mouse Cage; 7 ½ in W x 11 ½ in L x 5 in H) (Ancare) with standard woodchip mouse bedding (NORTHEASTERN PRODUCTS CORP., Aspen Chip)
Procedure
- Single house mice in proper mouse caging (see Equipment) under controlled conditions (see Materials and Reagents). Conditions should comply with the Guide for the Care and Use of Laboratory Animals, 8th ed. (https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf). Allow mice to acclimate for 7 d under these conditions.
- Setup 2-bottle choice test for baseline conditions (Figure 1).
- Fill 50 ml centrifuge tubes to ~40 ml with RO water and apply sipper cap.
- Invert tubes and allow air bubble in sipper tube to rise.
- Make sure the water line is below the conical tapered end when inverted.
- Label each tube (e.g., “A” & “B” in permanent marker) and mark location of water line using a permanent marker.
- Alternatively, the initial bottle weight can be recorded.
- Place the 2 inverted tubes into wire lid, with minimal shaking to avoid dripping.
- Both bottles should be placed on one side of the divided wire rack, leaving the other side for food.
- Make sure that the rubber stopper is flushed with caging and that steel sipper tubes extend below wire cage lid (Figure 1).
- Avoid water leakage from sipper tubes when replacing cage on rack.
- Put food chow on the other side of the wire lid.
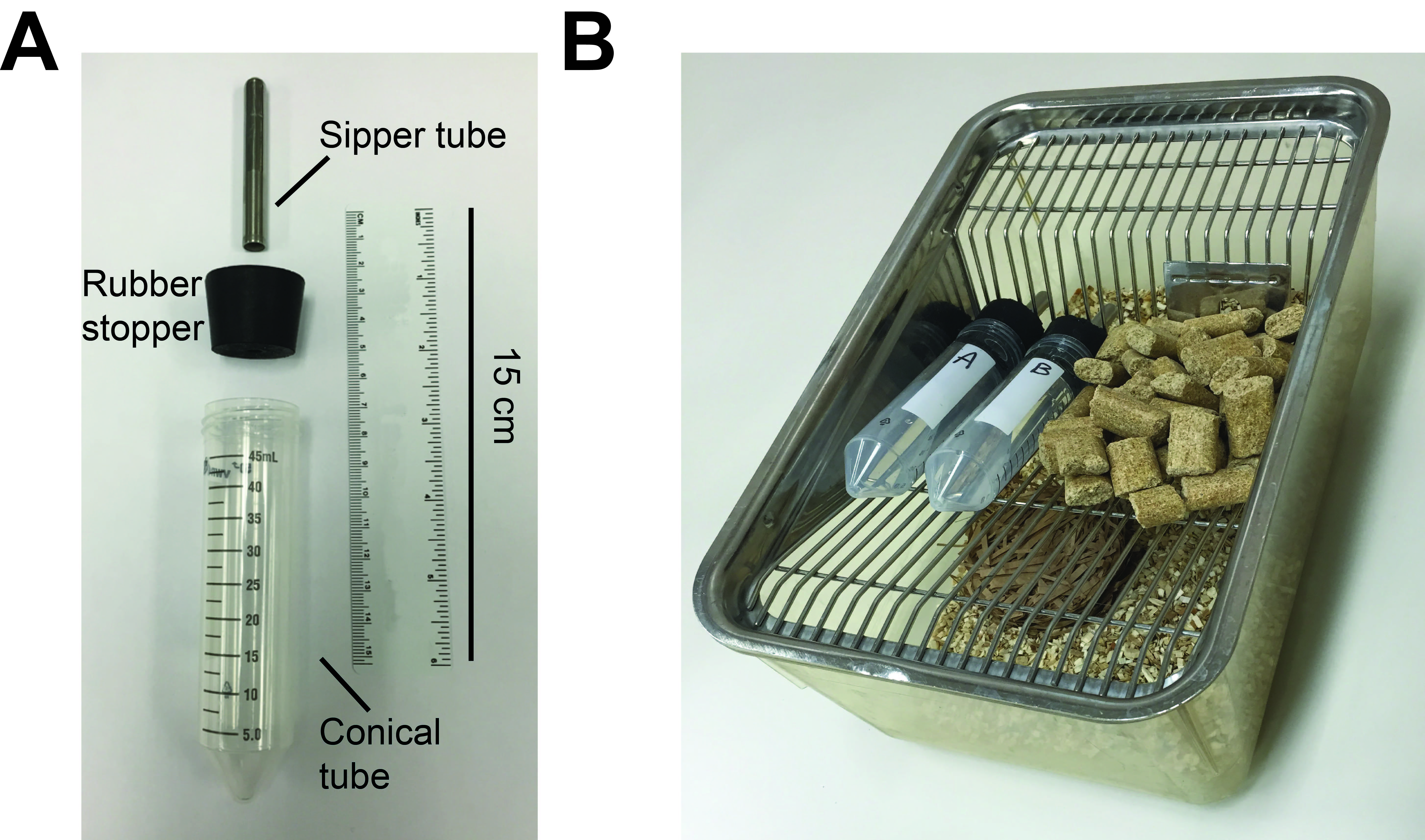
Figure 1. Picture of 2-bottle choice procedure setup in home cages. A. Sipper bottles separated out into its components consisting of metal sipper tube, rubber stopper (which the sipper tube is placed into via a hole in the center), and 50 ml conical centrifuge tube. B. Picture of 2-bottle setup in a home cage. The bottles are filled with solutions and place atop the wire mesh cage lid, with the metal sippers facing into the cage. - Fill 50 ml centrifuge tubes to ~40 ml with RO water and apply sipper cap.
- Assess baseline water consumption daily for 4 d. To assess consumption, weigh bottle or mark volume level.
- If conducting weight measurements, record pre- and post-consumption weights of the tube (1 g = 1 ml of water).
- For volume measurements, record volume levels on tube using a permanent marker.
- Different colored markers for each day are recommended.
- Do not invert tubes (where sipper cap is upright) when marking volume, as this allows air into the sipper and can cause dripping.
- Monitor for any problems with liquid flow and observe for signs of spillage, e.g., wet bedding, below the sipper tube.
- Switch positions (left vs right) of tubes daily.
- If conducting weight measurements, record pre- and post-consumption weights of the tube (1 g = 1 ml of water).
- Setup 2-bottle choice test for sucrose preference.
- Dissolve sucrose in RO water.
- Determination of sucrose concentration (% w/v; Figure 2) will require preliminary experiments using an initial dose-response curve (0.25-2%) to ascertain optimal sucrose preference necessary based on any a priori hypothesis.
- For example, if your hypothesized outcome indicates a likely reduction in preference, a greater concentration is recommended. However, if you are expecting an increase in preference, lesser concentrations may be more appropriate.
- Fill one tube (e.g., one marked “A”) with fresh RO water and the other (“B”) with the sucrose solution. Place tubes on wire lid as previously described.
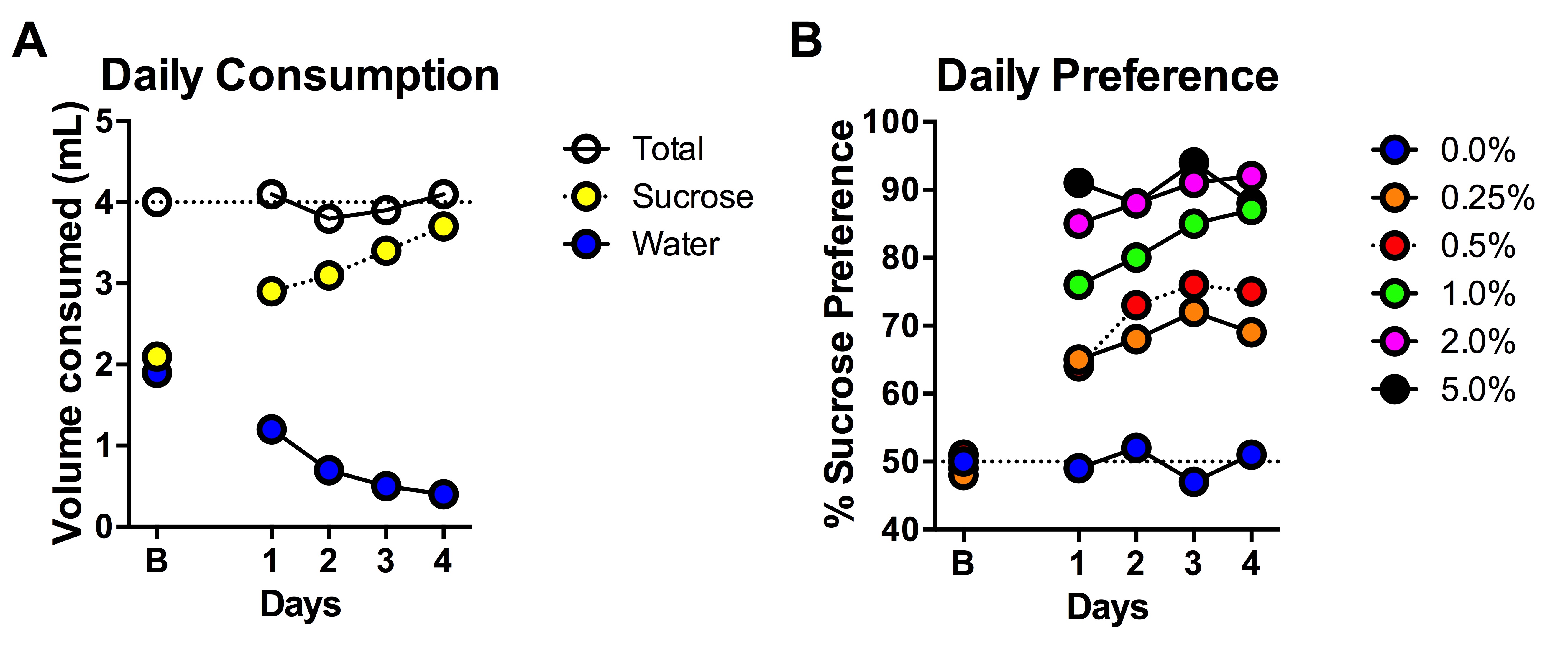
Figure 2. An illustration of preference for sucrose in the 2-bottle choice test. A. Sucrose preference measured by raw volume consumed; B. Sucrose preference measured by % preference for sucrose. - Dissolve sucrose in RO water.
- Assess water and sucrose solution consumption daily for 4 d. Record weights and/or volume of each tube. Monitor for any problems with liquid flow or spillage, e.g., wet bedding, below the sipper tube. Switch positions (left vs right) of tubes daily.
- Apply volume formula (see Notes) to determine consumption. Assess raw weight and/or volume consumed, and % preference for the sucrose-containing solution.
Notes
- Side bias
It is likely that some mice will develop bias for one side or the other, resulting in an increased consumption for one tube. Therefore, bottle positions within each cage are switched daily to avoid false sucrose preference resulting from side bias. Alternatively, if bias is a problem, tubes can be moved to the middle of the wire top and food chow placed on either side. - Volume formula
Volume of liquid consumed can be determined based on vertical markings on the side of the cylinder. This derives from the tube dimensions and distance between daily marks, using the volume of a cylinder equation:
V = π⋅ r2 ⋅ h
Where V = volume (in cm3, which is equivalent to ml), π ≈ 3.1416, r = radius (half the interior diameter in cm), and h = distance between daily markings (in cm). It is important to utilize only the cylindrical portions of the 50 ml tube, and avoid any fluid in the conical portion, as the volume formula differs there. - Animal considerations
It is important to conduct preliminary studies in order to characterize the behavioral phenotype with particular equipment, environment, and animals. This is especially true in the case of varying mouse strains, either inbred or outbred, or if mice have been surgically or behaviorally manipulated prior to sucrose preference testing. All of these factors can affect baseline preference. In addition, some labs prefer to pre-handle mice for 5-7 d and singly house the animals for a period of 5-7 d prior to preference testing.
Acknowledgments
This behavioral procedure was adapted from previously published studies (Krishnan et al., 2007; Robison et al., 2014) and was performed by our group as described (Eagle et al., 2015). This work was supported by the Whitehall Foundation (AJR; 2013-08-43), the Multidisciplinary Training in Environmental Toxicology training grant (ALE; T32-ES007255), and a 2014 NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (ALE).
References
- Barrot, M., Olivier, J. D., Perrotti, L. I., DiLeone, R. J., Berton, O., Eisch, A. J., Impey, S., Storm, D. R., Neve, R. L., Yin, J. C., Zachariou, V. and Nestler, E. J. (2002). CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A 99(17): 11435-11440.
- Bogucka-Bonikowska, A., Baran-Furga, H., Chmielewska, K., Habrat, B., Scinska, A., Kukwa, A., Koros, E., Kostowski, W., Polanowska, E. and Bienkowski, P. (2002). Taste function in methadone-maintained opioid-dependent men. Drug Alcohol Depend 68(1): 113-117.
- Eagle, A. L., Gajewski, P. A., Yang, M., Kechner, M. E., Al Masraf, B. S., Kennedy, P. J., Wang, H., Mazei-Robison, M. S. and Robison, A. J. (2015). Experience-dependent induction of hippocampal DeltaFosB controls learning. J Neurosci 35(40): 13773-13783.
- Hwa, L. S., Chu, A., Levinson, S. A., Kayyali, T. M., DeBold, J. F. and Miczek, K. A. (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35(11): 1938-1947.
- Institute of Laboratory Animal Resources (U.S.) (2011). Guide for the care and use of laboratory animals, 8th edition. Washington DC: National Academy of Sciences.
- Janowsky, D. S., Pucilowski, O. and Buyinza, M. (2003). Preference for higher sucrose concentrations in cocaine abusing-dependent patients. J Psychiatr Res 37(1): 35-41.
- Kampov-Polevoy, A., Garbutt, J. C. and Janowsky, D. (1997). Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry 154(2): 269-270.
- Krishnan, V., Han, M. H., Graham, D. L., Berton, O., Renthal, W., Russo, S. J., Laplant, Q., Graham, A., Lutter, M., Lagace, D. C., Ghose, S., Reister, R., Tannous, P., Green, T. A., Neve, R. L., Chakravarty, S., Kumar, A., Eisch, A. J., Self, D. W., Lee, F. S., Tamminga, C. A., Cooper, D. C., Gershenfeld, H. K. and Nestler, E. J. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131(2): 391-404.
- Pothion, S., Bizot, J. C., Trovero, F. and Belzung, C. (2004). Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res 155(1): 135-146.
- Robison, A. J., Vialou, V., Sun, H. S., Labonte, B., Golden, S. A., Dias, C., Turecki, G., Tamminga, C., Russo, S., Mazei-Robison, M. and Nestler, E. J. (2014). Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology 39(5): 1178-1186.
- Wallace, D. L., Vialou, V., Rios, L., Carle-Florence, T. L., Chakravarty, S., Kumar, A., Graham, D. L., Green, T. A., Kirk, A., Iniguez, S. D., Perrotti, L. I., Barrot, M., DiLeone, R. J., Nestler, E. J. and Bolanos-Guzman, C. A. (2008). The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci 28(41): 10272-10277.
- Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134(4): 319-329.
- Willner, P., Muscat, R. and Papp, M. (1992). Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16(4): 525-534.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Eagle, A. L., Mazei-Robison, M. and Robison, A. J. (2016). Sucrose Preference Test to Measure Stress-induced Anhedonia. Bio-protocol 6(11): e1822. DOI: 10.21769/BioProtoc.1822.
- Eagle, A. L., Gajewski, P. A., Yang, M., Kechner, M. E., Al Masraf, B. S., Kennedy, P. J., Wang, H., Mazei-Robison, M. S. and Robison, A. J. (2015). Experience-dependent induction of hippocampal DeltaFosB controls learning. J Neurosci 35(40): 13773-13783.
Category
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link