- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Arabidopsis Leaf Explant Culture
Published: Vol 5, Iss 22, Nov 20, 2015 DOI: 10.21769/BioProtoc.1654 Views: 12982
Reviewed by: Tie LiuTeresa LenserAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Ethylene Production in Leaf and Bud Tissue of the Subtropical Tree Crop Litchi (Litchi chinensis Sonn.) Using Gas Chromatography and Flame Ionization Detection
Regina B. Cronje and Arnoldus J. Jonker
Mar 20, 2023 1547 Views

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1941 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1885 Views
Abstract
In this protocol, Arabidopsis leaf explant culture is described using an adaptation of a previous method (Hu et al., 2000). Cells from the cut edges of leaf explant are able to proliferate and subsequently form calli on the callus induction medium, in which is supplemented with 2,4-D and 6-benzyl aminopurine [6-BA]. 2,4-D, one of the artificial auxin, is able to promote cell mitosis at low concentration. 6-BA, the first generation of synthetic cytokinin, plays an important role in plant cell division. 2,4-D in combination with 6-BA can effectively induce callus formation (Rashmi and Trivedi, 2014). The aim of this protocol is to analyze cell division competence of Arabidopsis plants with different genotypes. This protocol can be modified and applied to culture explants from other types of plant tissues, such as root and stem.
Keywords: Leaf explantMaterials and Reagents
- Plastic Petri dish
- Parafilm
- Millex-GP Filter Unit 0.22 μm (Merck Millipore Corporation, model: R4AA41572 )
- 2-3 weeks old sterile Arabidopsis plants (Murashige & Skoog solid medium grown)
- Murashige & Skoog medium (Duchefa Biochemie, catalog number: P11293.01 )
- Phytagel (Sigma-Aldrich, catalog number: MFCD00131909 )
- NaOH
- Sucrose
- 2, 4-dichlorophenoxyacetic acid (2, 4-D) (Sigma-Aldrich, catalog number: D7299 ) (see Recipes)
- 6-benzylaminopurine (6-BA) (Sigma-Aldrich, catalog number: 83488 ) (see Recipes)
- Murashige & Skoog solid medium (see Recipes)
- Callus induction medium (see Recipes)
Equipment
- Arabidopsis growth chamber
- Magnetic stirrer and stirring bar
- pH meter
- Scissors
- Tweezers
- Flow cabinet
Procedure
- Preparation of sterile Arabidopsis plants
- Prepare the Murashige & Skoog (MS) solid medium.
- Autoclave the Murashige & Skoog (MS) solid medium and pour it into Petri dishes when the temperature of the bottle reaches 50 °C.
- Put the Arabidopsis seeds in the 30% 84 bleach for 10 min for sterilization. Discard the bleach and wash the seeds with sterilized water for three times.
- Place 10~15 sterilized Arabidopsis seeds onto each Petri dish with the MS solid medium, and seal the Petri dishes with parafilm to keep sterile and moist.
- Store the Petri dishes in darkness at 4 °C for 2 to 4 d for vernalization.
- Transfer the Petri dishes to Arabidopsis growth chamber at 22 °C under a long-day condition (16 h light/8 h dark).
- Culture the Arabidopsis plants for about 2 to 3 weeks until the width of leaves reaches 3-5 mm (Figure 1).
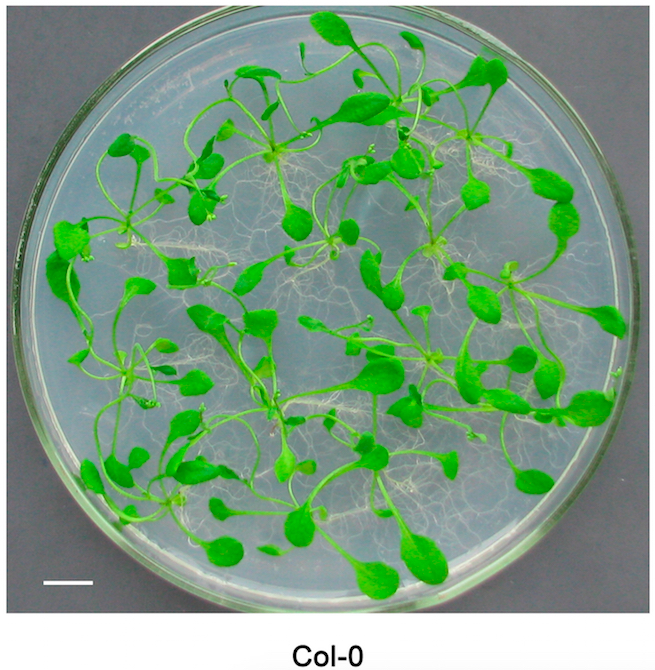
Figure 1. Arabidopsis plants culturing on the MS solid medium for 3 weeks. Bar = 1 cm.
- Prepare the Murashige & Skoog (MS) solid medium.
- Preparation of callus induction medium
- Prepare and autoclave the MS solid medium.
- Add 100 µl of 1 mg/L 2,4-D and 1 mg/L 6-BA into the MS solid medium, named callus induction medium, when the temperature of the bottle reaches 50 °C. Ensure that the performance is done in a flow cabinet.
- Pour the callus induction medium into Petri dishes. Ensure that the performance is done in a flow cabinet.
- Prepare and autoclave the MS solid medium.
- Leaf explant culture
- Ensure that all the performances from steps C11-13 are done in the flow cabinet.
- Cut across the leaf and midvein with sterilized scissors to yield small strips, which are about 5 mm in length and 2 mm in width, including the midvein going across the width. Ensure that similar mature rosette leaves are selected for the experiments.
- Select leaf strips in similar size and transfer them to the same callus induction medium with sterilized tweezers (Figure 2).

Figure 2. Arabidopsis leaf strips. Bar = 3 mm. - Seal the Petri dishes with parafilm to keep sterile and moist.
- Store the Petri dishes at 22 °C in darkness for 1 to 2 weeks.
- Observe the calli and take photographs (Figure 3).
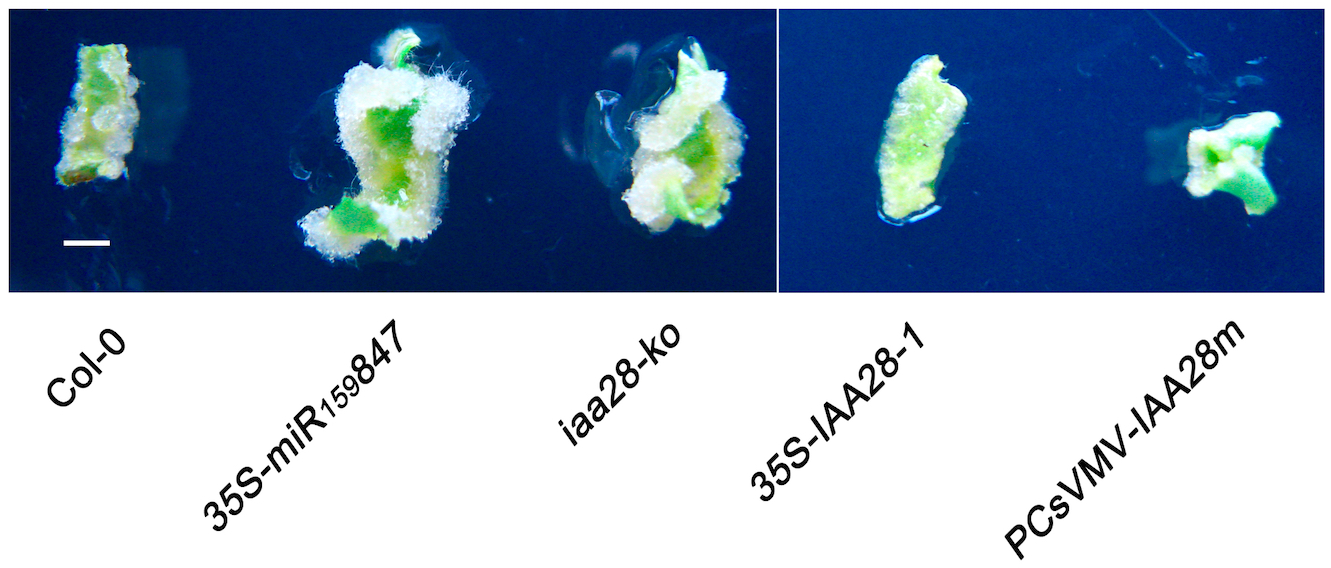
Figure 3. Callus growth in leaf explants (Wang and Guo, 2015). Callus growth in leaf explants of Col-0, 35S-miR159847 transgenic (lines 8), iaa28-ko mutant, and 35S-IAA28-1 and PCsVMV-IAA28m transgenic plants. The explants were photographed on day 7 without changing the medium. Bar = 2 mm.
- Ensure that all the performances from steps C11-13 are done in the flow cabinet.
Recipes
- 1 mg/ml 2,4-D
Dissolve 20 mg 2,4-D power in 5 ml ethanol, and while stirring, gently add water to 20 ml
Subsequently, sterilize it by filtration through a Millex-GP Filter Unit (0.22 µm), in the flow cabinet
Stored at -20 °C - 1 mg/ml 6-BA
Dissolve 20 mg 6-BA power in 5 ml 0.1 M NaOH, and while stirring, gently add water to 20 ml
Subsequently, sterilize it by filtration through a Millex-GP Filter Unit (0.22 µm) in the flow cabinet
Stored at -20 °C - Murashige & Skoog solid medium (1 L)
4.4 g Murashige & Skoog medium
30 g sucrose
3.5 g phytagel
Adjust pH to 5.9, using 1 M NaOH - Callus induction medium (1 L)
Murashige & Skoog solid medium
100 µl 1 mg/L 2,4-D
100 µl 1 mg/L 6-BA
Acknowledgments
This protocol was developed from the following published paper: Hu et al. (2000). This research was supported by the Ministry of Science and Technology (Grant2014CB138402).
References
- Hu, Y., Bao, F. and Li, J. (2000). Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J 24(5): 693-701.
- Rashmi, R. and Trivedi, M. P. (2014). Effect of various growth hormone concentration and combination on callus induction, nature of callus and callogenic response of Nerium odorum. Appl Biochem Biotechnol 172(5): 2562-2570.
- Wang, J. J. and Guo, H. S. (2015). Cleavage of INDOLE-3-ACETIC ACID INDUCIBLE28 mRNA by microRNA847 upregulates auxin signaling to modulate cell proliferation and lateral organ growth in Arabidopsis. Plant Cell 27(3): 574-590.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, J., Zhang, L. and Guo, H. (2015). Arabidopsis Leaf Explant Culture. Bio-protocol 5(22): e1654. DOI: 10.21769/BioProtoc.1654.
Category
Plant Science > Plant physiology > Plant growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












