- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Phosphorylation Assay of Putative Blue-light Receptor Phototropins Using Microsomal and Plasma-membrane Fractions Prepared from Vallisneria Leaves
Published: Vol 5, Iss 21, Nov 5, 2015 DOI: 10.21769/BioProtoc.1647 Views: 9403
Reviewed by: Samik BhattacharyaSam-Geun KongAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optimized Isolation of Lysosome-Related Organelles from Stationary Phase and Iron-Overloaded Chlamydomonas reinhardtii Cells
Jiling Li and Huan Long
Nov 20, 2024 1756 Views

Isolation and Biophysical Characterization of Extracellular Vesicles From Hairy Root Cultures
Marisa Conte [...] Alfredo Ambrosone
Mar 5, 2025 2203 Views

Rapid Miniprep of Intact Chloroplasts from Arabidopsis thaliana Leaves
Brenda A. Carranza-Correa [...] Manuel Gutiérrez-Aguilar
May 20, 2025 2637 Views
Abstract
An aquatic angiosperm Vallisneria (Alismatales: Hydrocharitaceae) has been used as an excellent experimental material over a century to study the light regulation of dynamic intracellular movements including chloroplast redistribution and cytoplasmic streaming (Senn, 1908; Seitz, 1987; Takagi, 1997). However, understanding of the molecular mechanisms lagged behind because of difficulty in applying modern techniques such as gene transformation to this plant. Especially, which kind of photoreceptors function in these intriguing responses has long been an unsolved topic. Recently, genes encoding plant-specific blue-light receptor phototropins were isolated in Vallisneria, for the first time from aquatic plants (Sakai et al., 2015). Phototropins were identified first as the photoreceptor for hypocotyl phototropism in Arabidopsis thaliana, and now known to regulate many responses including chloroplast photorelocation movements in various plant species (Christie, 2007). Phototropins are localized mainly on the plasma membrane and their auto-phosphorylation induced by blue light is the critical step of signal transduction pathway (Sakamoto and Briggs, 2002; Kong et al., 2006; Kong et al., 2013; Inoue et al., 2010). Here we describe a protocol for in vitro protein phosphorylation assay using crude-microsomal and plasma-membrane-enriched fractions of Vallisneria, which enabled us to verify the presence of phototropins and characterize their auto-phosphorylation responses. After these analyses, Sakai et al. (2015) proposed that Vallisneria phototropins mediate the high-intensity-blue-light-induced chloroplast avoidance response.
Keywords: Crude microsomal fractionMaterials and Reagents
- Commonly-used medical cotton gauze
- 0.2 ml tube
- Vallisneria plants
Note: Young plants of Vallisneria of 20-30 cm long were purchased at a tropical-fish store and cultured to be grown over 100 cm in buckets (20 L) filled with tap water and with a layer of soil at the bottom (Figure 1A). The plants were grown under 12 h light/12 h dark regime at 20-24 °C. - Dextran (from Leuconostoc ssp., Mr ~500,000) (Fluka, catalog number: 31392 )
Note: Currently, it is “Sigma-Aldrich, catalog number: 31392 ”. - Polyethylene glycol (Sigma-Aldrich, catalog number: P-3640 )
- SDS-PAGE
- Deionized water
- 1% Triton X-100 (Sigma-Aldrich, catalog number: T-9284 )
- 50 mM MOPS-KOH (pH7.6) (DOJINDO, catalog number: 343-01805 )
- 300 mM sucrose (Wako Pure Chemical Industries, Siyaku, catalog number: 196-00015 )
- 10 mM ethylene glycol bis (2-aminoethyl ether)-N,N,N’,N’,-tetraacetic acid (EGTA) (DOJINDO, catalog number: 342-01314 )
- 5 mM ethylenediaminetetraacetic acid (EDTA) (DOJINDO, catalog number: 345-01865 )
- 10 μg/ml dibutylhydroxytoluene (Tokyo Chemical Industry, catalog number: D0228 )
- 1% casein (Nacalai tesque, catalog number: 073-19 )
- 5 mM K2S2O5 (Wako Pure Chemical Industries, Siyaku, catalog number: 161-03345 )
- 1 mM dithiothreitol (DTT) (Wako Pure Chemical Industries, catalog number: 048-29224 )
- 2.5 mg/ml pepstatin A (Sigma-Aldrich, catalog number: P-4265 )
- 2.5 mg/ml aprotinin (Sigma-Aldrich, catalog number: A-4529 )
- 20 mg/ml EDTA-washed Polyvinylpolypyrolidone (Sigma-Aldrich, catalog number: P-6755 )
- MOPS-KOH (DOJINDO, catalog number: 345-02225 )
- 25 mM MgCl2 (Wako Pure Chemical Industries, Siyaku, catalog number: 135-00165 )
- 250 mM KCl (Wako Pure Chemical Industries, Siyaku, catalog number: 163-03545 )
- 40% glycerol (Wako Pure Chemical Industries, Siyaku, catalog number: 075-00616 )
- 20% 2-mercaptoethanol (Sigma-Aldrich, catalog number: A-6365 )
- A small amount of bromophenol blue (Wako Pure Chemical Industries, Siyaku, catalog number: 021-02911 )
- 0.5 M Tris-HCl (pH 6.8) (Sigma-Aldrich, catalog number: 252859 )
- Homogenizing medium (see Recipes)
- Buffer A (see Recipes)
- Buffer B (see Recipes)
- 5x phosphorylation buffer (see Recipes)
- ATP mixture (see Recipes)
- 4x SDS buffer (see Recipes)
Equipment
- Blade (Lion Office Products Corp., model: L-300 )
- Polytron homogenizer (Kinematica AG, model: PT-35/ 2ST”OD” )
- Centrifuge
- Ultracentrifuge
- Teflon homogenizer (10 ml) (Ikemoto Scientific Technology, model: 812-771-04 )
- Lighting system (Sugiura Lab, model: FI-150T )
- Cut-off filter (KenkoTokina Corporation, model: Y-44 )
- Interference filter (KenkoTokina Corporation, model: BP-45 )
- Autoradiography (Fujifilm Corporation)
Procedure
- Plant material and pretreatment of specimens
Young plants of Vallisneria of 20-30 cm long were purchased at a tropical-fish store and cultured to be grown over 100 cm in buckets (20 L) filled with tap water and with a layer of soil at the bottom (Figure 1A). The plants were grown under 12 h light/12 h dark regime at 20-24 °C. The light source was a bank of 20 W fluorescent lamps (50 μmol m-2 s-1). - Preparation of crude microsomal (CM) fraction and plasma membrane-enriched (PM) fraction
- At the end of the light period, 40 g of the healthy fresh leaves were harvested into tap water and kept in complete darkness for 12-16 h.
Note: Before use, snip off unhealthy part from the leaves. - The leaves were chopped on ice with a blade (Figure 1B). These small leaf pieces were homogenized with a Polytron homogenizer (Figure 1C) in 120 ml of a homogenizing medium.
- The homogenate was filtrated through six sheets of gauze (Figure 1D) and centrifuged (8,000 x g, 15 min, 4 °C).
- The supernatant (Figure 1E) was ultracentrifuged at 156,000 x g for 1 h at 4 °C.
- The pellet was suspended with a Teflon homogenizer in Buffer B (Figure 1F), followed by another ultracentrifugation at 156,000 x g for 1 h at 4 °C.
- The pellet (Figure 1G) was washed with buffer A and then re-suspended into a small volume (usually 6 ml) of the fresh buffer A, designated as a CM fraction. When the CM fraction was directly used for phosphorylation assay, it was washed and suspended with buffer B, not buffer A.
- A highly purified PM fraction was isolated from the CM fraction by applying aqueous polymer two-phase partitioning (Figure 1H-I) using Dextran from Leuconostoc ssp. Mr ~500,000 and polyethylene glycol (Yoshida et al., 1983; Takagi et al., 1988; Harada et al., 2002).
- After the final ultracentrifugation of the polyethylene-glycol-enriched upper phase, the pellet was washed with buffer B and then re-suspended into a small volume of the fresh buffer, designated as a PM fraction.
Note: All the procedures were carried out on ice at 0-4 °C under dim red light (0.1 μmol m-2 s-1). The total protein contents in the CM and PM fractions were determined by Bradford assay. The CM and PM fractions were kept in darkness on ice until use.

Figure 1. Preparation steps of CM and PM fractions. A. Vallisneria plants cultured in buckets. B. Leaves were chopped on ice. C. Leaves were further homogenized with a Polytron homogenizer. D. The homogenate was filtrated through six sheets of gauze. E. The filtrated homogenate before low-speed centrifugation (8,000 x g, 15 min) to remove debris such as cell wall. F. The pellet after the first ultracentrifugation (156,000 x g, 1 h) was suspended with a Teflon homogenizer. G. The pellet after the second ultracentrifugation (156,000 x g, 1 h). H and I. The plasma-membrane-enriched upper phase was separated from the lower phase after the first (H) and second (I) two-phase partitioning. - At the end of the light period, 40 g of the healthy fresh leaves were harvested into tap water and kept in complete darkness for 12-16 h.
- In vitro phosphorylation assay
- The CM and PM fraction were suspended in the reaction mixture as described below.
Reaction mixture (17.5 μl for each reaction)CM and PM fraction containing 20 μg protein x μl 5x phosphorylation buffer 4 μl 1% Triton X-100 1 μl Deionized water 17.5-x μl - The reaction mixture put into a 0.2 ml tube was irradiated with blue light (450 nm) for a defined period of time (usually 0-300 sec) using a lighting system equipped with a fiber scope, a cut off filter, and an interference filter (Figure 2A).
- Immediately after the end of the irradiation, the reaction mixture was mixed with ATP mixture (2.5 μl) containing radioactive ATP and incubated for 2 min to allow phosphorylation reaction. The reaction was terminated by the addition of 4x SDS buffer (7 μl).
Note: Steps C1-3 were carried out on ice under dim red light (0.1 μmol m-2 s-1) in the dark room to avoid any non-specific excitation of photoreceptors by background light. - The sample was boiled for 2 min and subjected to SDS-PAGE in a 10.0% (w/v) gel. Separated polypeptides on the gel were stained once with Coomassie Brilliant Blue to visualize the protein profiles (Figure 2B, the left panel).
Note: In each lane, 20 μg of proteins were loaded. - Autoradiography was carried out by exposing the de-stained and dried-up gels to an imaging plate for 0.5-1 h at room temperature (Figure 2B, the right panel).
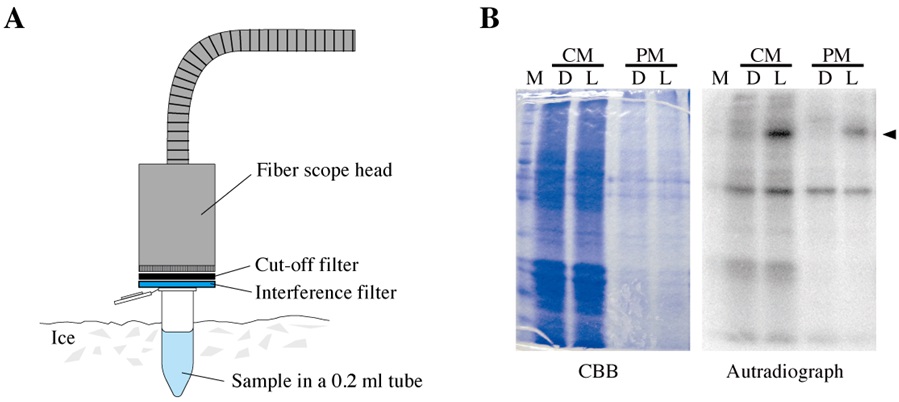
Figure 2. Irradiation and autoradiography. A. Irradiation of a sample in a 0.2-ml tube on ice using a lighting system equipped with a fiber scope, a cut-off filter and an interference filter. B. A dried-up gel stained with Coomassie Brilliant Blue (CBB; the left panel) and its autoradiograph (the right panel). Before (D) and after (L) the actinic irradiation, the protein-phosphorylation levels were examined in a crude microsomal fraction (CM) and a plasma-membrane-enriched fraction (PM). M; molecular size markers, arrowhead; radioactive signal of phosphorylation of Vallisneria phototropins.
- The CM and PM fraction were suspended in the reaction mixture as described below.
Recipes
- Homogenizing medium
50 mM MOPS-KOH (pH 7.6)
300 mM sucrose
10 mM ethylene glycol bis (2-aminoethyl ether)-N,N,N’,N’,-tetraacetic acid (EGTA)
5 mM ethylenediaminetetraacetic acid (EDTA)
10 μg/ml dibutylhydroxytoluene
1% casein
5 mM K2S2O5
1 mM dithiothreitol (DTT)
2.5 mg/ml pepstatin A
2.5 mg/ml aprotinin
20 mg/ml EDTA-washed Polyvinylpolypyrolidone
Note: Contents of the medium were mixed with a magnetic stirrer at 4 °C overnight (12-16 h), except K2S2O5, DTT, pepstatin A, aprotinin, and EDTA-washed Polyvinylpolypyrolidone, which were added immediately before use. Polyvinylpolypyrolidone was previously washed with 5-mM EDTA, filtrated with a filter paper and dried up on the filter paper at room temperature for 1-2 days. - Buffer A
10 mM K-phosphate (pH 7.8)
250 mM sucrose - Buffer B
5 mM MOPS-KOH (pH7.6)
250 mM sucrose
0.1 mM DTT - 5x phosphorylation buffer
250 mM Mops-KOH (pH 7.0)
25 mM MgCl2
250 mM KCl
5 mM DTT - ATP mixture (2.5 μl for each reaction)
10 mCi/ml [γ-32P] ATP0.5 μl
20 μM ATP 2 μl - 4x SDS buffer
8% sodium dodecyl sulfate (SDS)
40% glycerol
20% 2-merchaptethanol
A small amount of bromophenol blue
0.5 M Tris-HCl (pH 6.8)
Acknowledgments
This protocol was modified from previous work by Takagi et al. (1988) and Harada et al. (2002). We thank Mr. Motoyuki Iida for taking pictures of culture facilities of Vallisneria plants (Figure 1A).
References
- Christie, J. M. (2007). Phototropin blue-light receptors. Annu Rev Plant Biol 58: 21-45.
- Harada, A., Okazaki, Y. and Takagi, S. (2002). Photosynthetic control of the plasma membrane H+-ATPase in Vallisneria leaves. I. Regulation of activity during light-induced membrane hyperpolarization. Planta 214(6): 863-869.
- Inoue, S., Takemiya, A. and Shimazaki, K. (2010). Phototropin signaling and stomatal opening as a model case. Curr Opin Plant Biol 13(5): 587-593.
- Kong, S. G., Suzuki, T., Tamura, K., Mochizuki, N., Hara-Nishimura, I. and Nagatani, A. (2006). Blue light-induced association of phototropin 2 with the Golgi apparatus. Plant J 45(6): 994-1005.
- Kong, S. G., Suetsugu, N., Kikuchi, S., Nakai, M., Nagatani, A. and Wada, M. (2013). Both phototropin 1 and 2 localize on the chloroplast outer membrane with distinct localization activity. Plant Cell Physiol 54(1): 80-92.
- Sakai, Y., Inoue, S., Harada, A., Shimazaki, K. and Takagi, S. (2015). Blue-light-induced rapid chloroplast de-anchoring in Vallisneria epidermal cells. J Integr Plant Biol 57(1): 93-105.
- Sakamoto, K. and Briggs, W. R. (2002). Cellular and subcellular localization of phototropin 1. Plant Cell 14(8): 1723-1735.
- Seitz K (1987) Light-dependent movement of chloroplsts in higher plant cell. ACTA PHYSIOLOGIAE PLANTARUM 9: 137-148
- Senn, G. (1908). Die Gestalts- und Lageveränderung der Pflanzen-Chromatophoren. Verlag von Wilhelm Engelmann, Leipzig (in Germany).
- Takagi, S., Yoshida, S. and Nagai, R. (1988). A Model for Cell Membrane Isoration Using Vallisneria Leaves. Methods in bryology. Proc Bryol Meth, 81-88.
- Takagi, S. (1997). Photoregulation of cytoplasmic streaming: cell biological dissection of signal transduction pathway. J Plant Res. 110: 299-303.
- Yoshida, S., Uemura, M., Niki, T., Sakai, A. and Gusta, L. V. (1983). Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol 72(1): 105-114.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sakai, Y., Inoue, S. and Takagi, S. (2015). In vitro Phosphorylation Assay of Putative Blue-light Receptor Phototropins Using Microsomal and Plasma-membrane Fractions Prepared from Vallisneria Leaves. Bio-protocol 5(21): e1647. DOI: 10.21769/BioProtoc.1647.
Category
Plant Science > Plant cell biology > Organelle isolation
Biochemistry > Protein > Modification
Cell Biology > Cell signaling > Phosphorylation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









