- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Observation of Chloroplast Movement in Vallisneria
Published: Vol 5, Iss 21, Nov 5, 2015 DOI: 10.21769/BioProtoc.1646 Views: 9882
Reviewed by: Samik BhattacharyaSam-Geun KongAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Using a Live Analysis System to Study Amyloplast Replication in Arabidopsis Ovule Integuments
Makoto T. Fujiwara [...] Ryuuichi D. Itoh
Jun 5, 2025 2557 Views

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2312 Views

A Simple Protocol for Periodic Live Cell Observation of Flagellate Stages in the Lichen Alga Trebouxia
Enrico Boccato [...] Mauro Tretiach
Jan 20, 2026 179 Views
Abstract
Chloroplasts accumulate to weak light and escape from strong light. These light-induced responses have been known from the 19th century (Böhm, 1856). Up to now, many scientists have developed different methods to investigate these dynamic phenomena in a variety of plant species including the model plant Arabidopsis thaliana, a terrestrial dicot (Wada, 2013). Especially, a serial recording to trace the position of individual chloroplast for the analysis of its mode of movement is critical to understand the underlying mechanism. An aquatic monocot Vallisneria (Alismatales: Hydrocharitaceae, Figure 1A) has contributed over a century to such investigation (Senn, 1908; Zurzycki, 1955; Seitz, 1967), because Vallisneria leaves have rectangular parallelepiped-shaped epidermal cells aligned orderly in a monolayer (Figure 1B), providing an excellent experimental system for microscopic studies. Here we describe a protocol for the up-to-date time-lapse imaging procedures to analyze Vallisneria chloroplast movement. Using this and prototype procedures, the relevant photoreceptor systems (Izutani et al., 1990; Dong et al., 1995; Sakai et al., 2015), association with actin cytoskeleton (Dong et al., 1996; Dong et al., 1998; Sakai and Takagi 2005; Sakurai et al., 2005), and regulatory roles of Ca2+ (Sakai et al., 2015) have been strenuously investigated.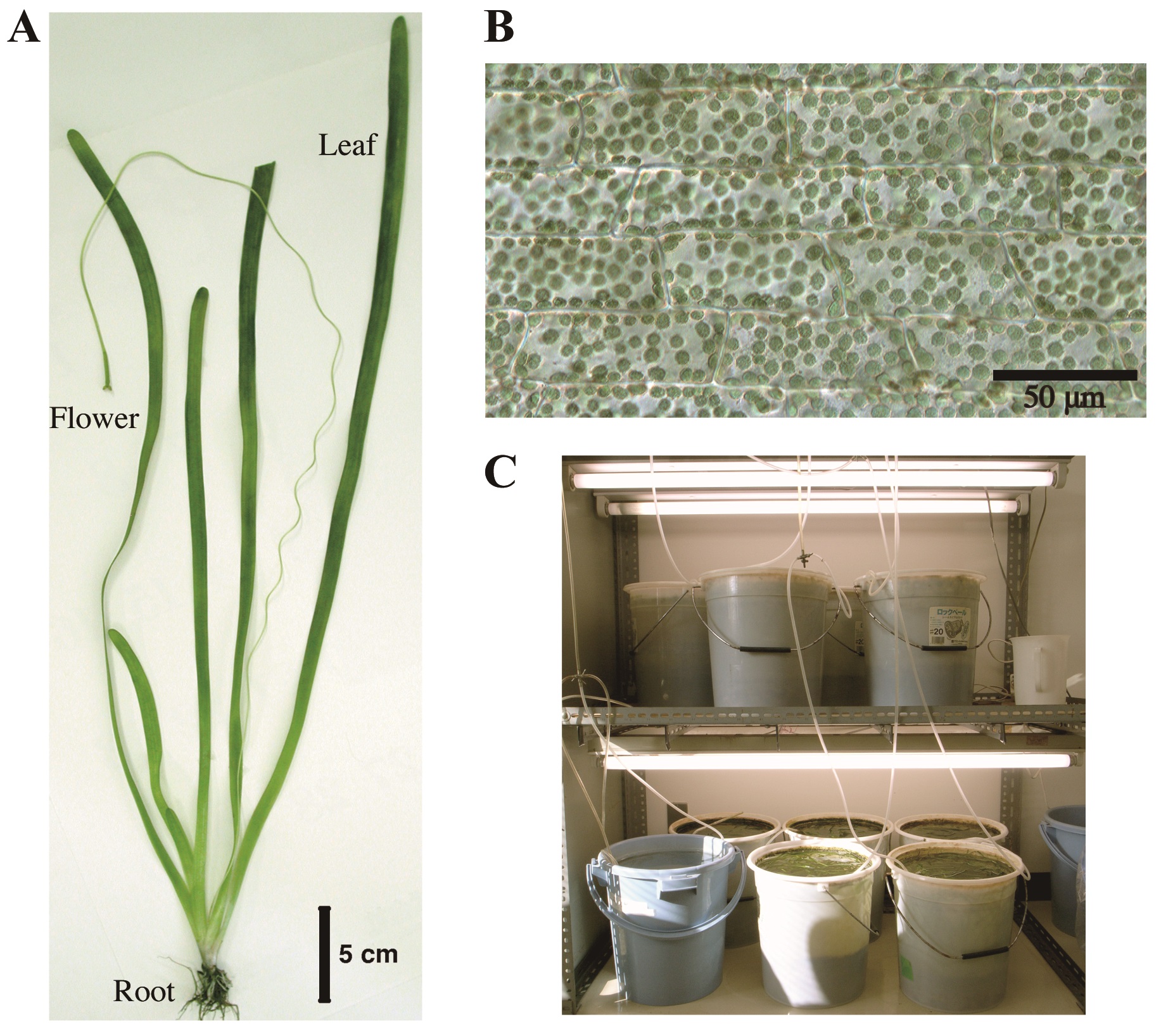
Figure 1. Vallisneria plant. A. Whole plant body; B. A bright-field image of adaxial epidermal cells containing a large number of chloroplasts; C. Culture facilities.
Materials and Reagents
- Petri dish
- Vallisneria plants
Note: Young plants of Vallisneria of 20-30 cm long were purchased at a tropical-fish store and cultured in buckets (20 L) filled with tap water and with a layer of soil at the bottom (Figure 1C). The plants were grown under 12 h light/12 h dark regime at 20-24 °C. - Vaseline (Wako Pure Chemical Industries, Siyaku, catalog number: 224-00165 )
- Potassium Chloride (KCl) (Wako Pure Chemical Industries, Siyaku, catalog number: 163-03545 )
- Sodium chloride (NaCl) (Nacalai tesque, catalog number: 31320-05 )
- Calcium Nitrate Tetrahydrate [Ca(NO3)2] (Wako Pure Chemical Industries, Siyaku, catalog number: 039-00735 )
- Magnesium Nitrate Hexahydrate [Mg(NO3)2] (Wako Pure Chemical Industries, Siyaku, catalog number: 134-00255 )
- PIPES (DOJINDO, catalog number: 345-02225 )
- Sodium hydroxide (NaOH) (Wako Pure Chemical Industries, Siyaku, catalog number: 197-02125 )
- Artificial pond water (APW) (see Recipes)
- 100x APW mixture (see Recipes)
- 200 mM PIPES-NaOH (see Recipes)
Equipment
- Fluorescent lamps (National, model: FL20S-PG )
- Vacuum pump (ULVAC KIKO, model: DTU-20 )
- Microscope (OLYMPUS, model: BX50 )
- Neutral-density filters (Fujifilm Corporation)
- Interference filter (Toshiba, model: KL-55 )
- Cut-off filter (Toshiba, model: O-54 )
- Quantum sensor (LI-COR Biosciences, model: LI-190SA )
- Data logger (LI-COR Biosciences, model: LI-1400 )
- Charge-coupled device camera (QImaging, model: RETIGA 2000RV )
Procedure
- Plant material and pretreatment of specimens
- Young plants of Vallisneria of 20-30 cm long were purchased at a tropical-fish store and cultured in buckets (20 L) filled with tap water and with a layer of soil at the bottom (Figure 1C). The plants were grown under 12 h light/12 h dark regime at 20-24 °C. The light source was a bank of 20 W fluorescent lamps (50 μmol m-2 s-1, Figure 1C). The following steps A2-7 were carried out at 20-24 °C.
- At the end of the light period, healthy leaf segments of 10 cm long taken between 30 and 40 cm from the base of shoots, which had grown over 100 cm, were cut into smaller pieces about 4 mm in length and were put into artificial pond water (APW).
- The air trapped in the intercellular spaces was evacuated using a desiccator connected with a rotary vacuum pump for 20 min.
- Each 4 mm length leaf piece was further cut open and separated into adaxial half and abaxial half (Figure 2A; Sakurai et al., 2005).
- Each adaxial half was floated in a separate vessel with fresh APW and kept under another cycle of 12 h light/12 h dark regime.
- The leaf piece was mounted on a glass slide with a coverslip so that the epidermal cells could be seen from above, and secured with white Vaseline (Figure 2B).
- Each specimen was immersed in fresh APW in a Petri dish (Figure 2B) and kept under weak white light (2 μmol m-2 s-1) from the fluorescent lamps for 6 h. They were further kept in complete darkness for another 12-20 h.
- Young plants of Vallisneria of 20-30 cm long were purchased at a tropical-fish store and cultured in buckets (20 L) filled with tap water and with a layer of soil at the bottom (Figure 1C). The plants were grown under 12 h light/12 h dark regime at 20-24 °C. The light source was a bank of 20 W fluorescent lamps (50 μmol m-2 s-1, Figure 1C). The following steps A2-7 were carried out at 20-24 °C.
- Time-lapse light microscopy for observation of chloroplast movement
- The dark-adapted specimen was irradiated with blue light (488 nm, 80 μmol m-2 s-1) on the stage of a microscope from above through the objective lens, using the epi-illumination system with a dichroic mirror and a mercury lamp (Figure 2C). The fluence rate of blue light was adjusted manually with neutral-density filters.
- The specimen was observed with dim green light (550 nm, 10 μmol m-2 s-1) produced by combination of an interference filter (the maximal transmission is 35%) and a cut-off filter, according to Izutani et al. (1990), applied from below. Although many of the plant photoreceptors are known to absorb light in green region, we confirmed that light of 550 nm at this fluence rate barely affects chloroplast movement and cytoplasmic motility in Vallisneria (Izutani et al., 1990; Takagi et al., 2003).
Note: The fluence rate of monochromatic light was measured with a quantum sensor and data logger. - Before and during the actinic blue-light irradiation, optical images were captured digitally with a charge-coupled device camera at 5-sec intervals (Figure 2C).
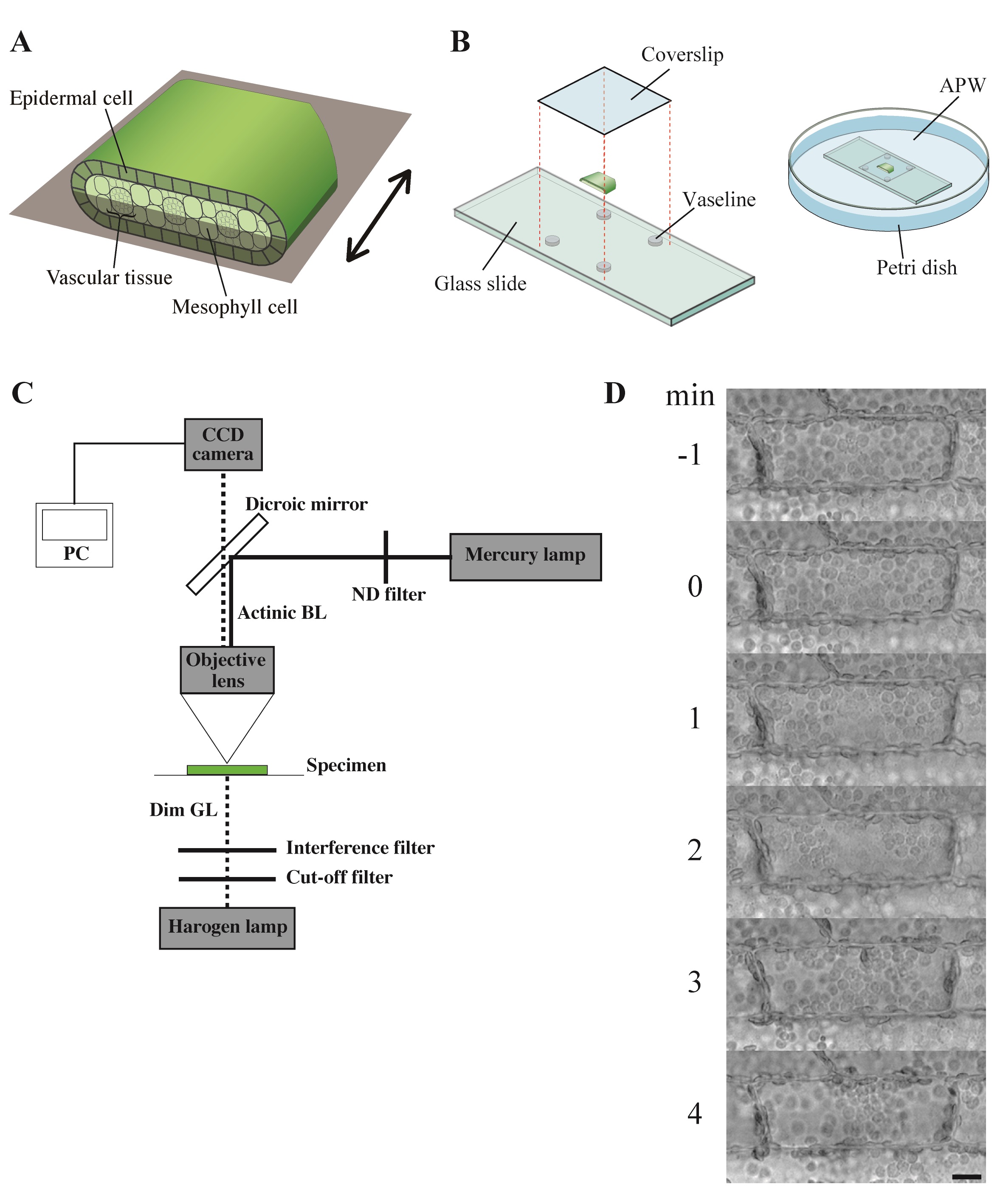
Figure 3. Observation of chloroplast movement. A. Scheme to prepare the adaxial leaf piece. Double-headed arrow; the longitudinal axis of leaf. Shadowed plane; cutting plane to make the adaxial and abaxial halves of leaf piece. B. Preparation of the specimen before the dark adaptation. APW; artificial pond water. C. Optical system. BL; blue light (488 nm, 80 μmol m-2 s-1), GL; green light (550 nm, 10 μmol m-2 s-1), ND; neutral density, CCD; charge-coupled device, PC; personal computer. D. Serial images of an epidermal cell arranged at 1-min intervals. At 0 min, the cell was exposed to blue light. Bar, 10 μm. - The number and position of chloroplasts on each digital image were recorded for movement analyses. A series of digital images can also be assembled into a movie (Video 1).Video 1. Avoidance movement of chloroplasts in Vallisneria. A series of 5-sec interval images for 1 min before and 19 min during the blue-light irradiation were stacked into a movie (12 frame per sec). Bright flash in the movie means the start of the irradiation. Bar, 10 μm.
- When necessary, before the start of observation, specimens were perfused with APW containing various kinds of chemicals, for example, inhibitors of photosynthetic electron transport, cytoskeleton-disrupting reagents, and intracellular-calcium-transient inhibitors. All the procedures were carried out under dim green light (0.03 μmol m-2 s-1).
- The dark-adapted specimen was irradiated with blue light (488 nm, 80 μmol m-2 s-1) on the stage of a microscope from above through the objective lens, using the epi-illumination system with a dichroic mirror and a mercury lamp (Figure 2C). The fluence rate of blue light was adjusted manually with neutral-density filters.
Notes
- Vallisneria leaf cells are extraordinarily sensitive to touch and chemicals. Such unintended stimuli should be excluded as much as possible.
Recipes
- Artificial pond water (APW)
APW could be prepared in large volume and stored at room temperature.
To prepare 10 L of APW, mix
100 ml 100x APW mixture
100 ml 200 mM PIPES-NaOH (pH 7.0)
800 ml of deionized water - 100x APW mixture
100x APW could be stored at 4 °C.
To prepare 1 L of 100x APW mixture, mix
3.73 g KCl (final conc. 50mM)
1.17 g NaCl (final conc. 20 mM)
1.64 g Ca(NO3)2 (final conc. 10 mM)
1.48 g Mg(NO3)2 (final conc. 10 mM) - 200 mM PIPES-NaOH (pH 7.0)
60.48 g PIPES solved in 1 L of deionized water, adjusted to pH 7.0 with NaOH
It can be stored at 4 °C.
Acknowledgments
We thank Mr. Motoyuki Iida for taking pictures of culture facilities of Vallisneria plants (Figure 1C).
References
- Böhm, J. A. (1856). Beiträge zur näheren Kenntnis des Chlorophylls. S. B. Akad. Wiss. Wien, Math.-nat. Kl. 22: 479-498.
- Dong, X. J., Takagi, S. and Nagai, R. (1995). Regulation of the orientation movement of chloroplasts in epidermal cells of Vallisneria: cooperation of phytochrome with photosynthetic pigment under low-fluence-rate light. Planta 197: 257-263.
- Dong, X. J., Ryu, J. H., Takagi, S. and Nagai, R. (1996) Dynamic changes in the organization of microfilaments assosiated wite the photocontrolled motility of chloroplasts in epidermal cells of Vallisneria. Protoplasma 195: 18-24.
- Dong, X. J., Nagai, R. and Takagi, S. (1998) Microfilaments anchor chloroplasts along the outer periclinal wall in Vallisneria epidermal cells through cooperation of Pfr and Photosynthesis. Plant & Cell Physiology 39: 1299-1306.
- Izutani, Y., Takagi, S. and Nagai, R. (1990). Orientation movements of chloroplasts in Vallisneria epidermal cells: Different effects of light at low- and high-fluence rate. Photochem Photobiol 51: 105-111.
- Sakai, Y. and Takagi, S. (2005). Reorganized actin filaments anchor chloroplasts along the anticlinal walls of Vallisneria epidermal cells under high-intensity blue light. Planta 221(6): 823-830.
- Sakai, Y., Inoue, S., Harada, A., Shimazaki, K. and Takagi, S. (2015). Blue-light-induced rapid chloroplast de-anchoring in Vallisneria epidermal cells. J Integr Plant Biol 57(1): 93-105.
- Sakurai, N., Domoto, K. and Takagi, S. (2005). Blue-light-induced reorganization of the actin cytoskeleton and the avoidance response of chloroplasts in epidermal cells of Vallisneria gigantea. Planta 221(1): 66-74.
- Seitz, K. (1967). Wirkungsspektren für die Starklichtbewegung der Chloroplasten, die Photodinese und die lichtabhängige Viskositätsänderung bei Vallisneria spiralis. Z Pflanzenphysiol 56:246-261.
- Senn, G. (1908). Die Gestalts- und Lageveränderung der Pflanzen-Chromatophoren. Verlag von Wilhelm Engelmann, Leipzig (in Germany).
- Takagi, S., Kong, S. G., Mineyuki, Y. and Furuya, M. (2003). Regulation of actin-dependent cytoplasmic motility by type II phytochrome occurs within seconds in Vallisneria gigantea epidermal cells. Plant Cell 15(2): 331-345.
- Wada, M. (2013). Chloroplast movement. Plant Sci 210: 177-182.
- Zurzycki, J. (1955). Chloroplasts arrangement as a factor in photosynthesis. Acta Soc Bot Pol 24: 27-63.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sakai, Y. and Takagi, S. (2015). Observation of Chloroplast Movement in Vallisneria. Bio-protocol 5(21): e1646. DOI: 10.21769/BioProtoc.1646.
Category
Plant Science > Plant cell biology > Cell structure
Plant Science > Plant physiology > Photosynthesis
Cell Biology > Cell imaging > Live-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










