- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Histochemical Staining of Silica Body in Rice Leaf Blades
Published: Vol 5, Iss 19, Oct 5, 2015 DOI: 10.21769/BioProtoc.1609 Views: 11310
Reviewed by: Tie LiuYuko KuritaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of the Humidity Effect on HR by Ion Leakage Assay
Musoki Mwimba and Xinnian Dong
Apr 5, 2019 6105 Views

Visualization of Actin Organization and Quantification in Fixed Arabidopsis Pollen Grains and Tubes
Xiaolu Qu [...] Shanjin Huang
Jan 5, 2020 5342 Views

Acidified Blue Ink-staining Procedure for the Observation of Fungal Structures Inside Roots of Two Disparate Plant Lineages
Jill Kowal [...] Sophie Lane
Oct 20, 2020 5030 Views
Abstract
Silicon (Si) is a biologically important element for plants in the order Poales (Yamamoto et al., 2011; Kido et al., 2015). In rice, Si is mainly deposited in the motor cells and the cell walls of the leaf epidermis. However, the molecular basis of this overall process has not been elucidated. Thus, we propose a protocol for the histochemical staining of the silica body based on specific hydrogen bonding between silanol group and the carboxylate group of crystal violet lactone (Ichimura et al., 2008), as described by Isa et al. (2010), but with minor modifications. This modified protocol can be used for observing Si accumulation during rice development.
Keywords: RiceMaterials and Reagents
- Rice (Oryza sativa L. cv. Nipponbare) plants were grown in a liquid medium supplemented with 1.5 mM SiO32- in a growth chamber at 28 °C under a 15/9 h light/dark cycle (light at 150 μ mol-2 s-1). This protocol is performed with the fifth leaf blade at foliar age 5.2 (Kido et al., 2015). The foliar age “m.n” is defined as those in which the mth leaf is fully expanded and the (m+1)th leaf is under development with a length of n/10 of the fully expanded-length
- Paraformaldehyde (Wako Pure Chemical Industries, catalog number: 162-16065 )
- Sodium cacodylate buffer (Nacalai Tesque, catalog number: 37238-25 )
- Agar powder (Nacalai Tesque, catalog number: 01028-85 )
- Phosphate Buffered Saline (PBS) (Takara Bio, catalog number: T900 )
- Ethanol (Wako Pure Chemical Industries, catalog number: 057-00456 )
- Molecular sieves (Sigma-Aldrich, catalog number: M6141 )
- Benzene (Nacalai Tesque, catalog number: 04017-35 )
- Crystal violet lactone (Tokyo Chemical Industry UK Ltd, catalog number: C0741 )
- PBS tablets (Takara Bio, catalog number: T900)
- Phosphate-buffered saline (PBS) (see Recipes)
- 100% ethanol solution (see Recipes)
- Crystal violet lactone solution (see Recipes)
Equipment
- Growth chamber (Nippon Medical & Chemical Instruments, model: LH220S )
- Stirrer Hotplate (Thermo Fisher Scientific, model: Fisher Scientific Isotemp )
- Diaphragm vacuum pump (Leybold-Heraeus, model: Divac 2.2L )
- Desiccator (SANPLATEC, model: PC-250K )
- Microwave oven (Sharp Electronics, model: RE-T12 )
- Paraffin dish (Greiner Bio-One GmbH, catalog number: 908177 )
- Leica VT1200S vibrating blade microtome (Leica Microsystems, model: VT1200S )
- Microscope slide (Matsunami Glass, catalog number: S-2123 )
- Cover slip (Matsunami Glass, catalog number: C024361 )
- Optical microscope (Leica Microsystems, model: DMRPX )
- CCD camera (QImaging, model: Retiga EXi )
Procedure
- Prepare the fixative by dissolving 0.4 g of paraformaldehyde powder in 10 ml of 20 mM sodium cacodylate buffer (pH 7.4). Heat this solution in a fume hood on the hotplate/stirrer to approximately 70 °C until the solution clears completely, and allow it to cool. The fixative mixture should be prepared immediately before use.
- Cut the rice leaf blade into pieces measuring approximately 2-3 mm in length (Figure 1A) and immediately immerse it in the fixative (the cut tissue will float on the fixative).
- The tissue/fixative is placed in a desiccator, which is connected to a diaphragm vacuum pump. The tissue/fixative is vacuum degasified for 5 min at room temperature using the vacuum pump before releasing the vacuum very slowly. Pull and release the vacuum again until the tissue sinks.
- Incubate overnight at 4 °C without vacuum.
- Dissolve 5.0 g agar powder in 100 ml of PBS using a microwave oven. Place the molten agar medium on the hotplate/stirrer, which must be preheated to 60 °C.
- Remove the fixative. Rinse the tissue three times with PBS. Pour the tissue into a paraffin dish.
- Cover the paraffin dish with molten agar medium. Arrange the leaf blade tissue in a regular array (Figure 1B and Figure 2A). Allow the agar to solidify for at least 15 min (Figure 1C).
- Glue the agar block, in which pieces of cut leaf blade tissues were placed upright, onto the stage of a Leica VT1200S vibrating blade microtome with a drop of instant glue (Konishi Aron Alpha) (Figure 1D-E and Figure 2B).
- Cut the agar-embedded tissue transversely, set upright in the agar block, with a thickness of 70 μm with the vibroslicer (Figure 1F-H and Figure 2C). Collect the transverse sections in PBS by tweezers.
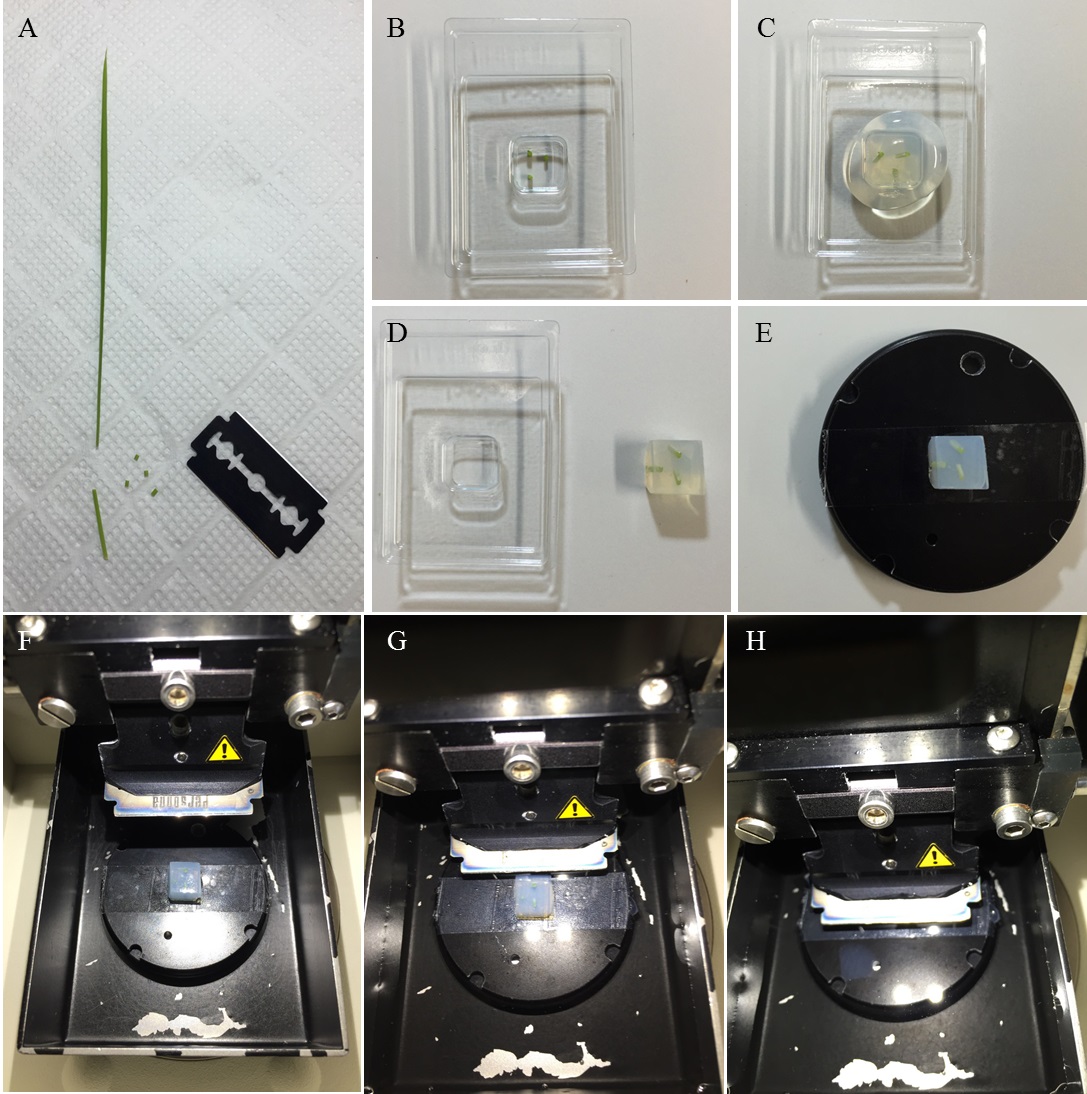
Figure 1. Section preparation. A. The fifth leaf blades cut with a razor blade. B-E. Embedding of pieces of rice blade tissues in agar. F-H. Sectioning with a vibroslicer equipped with a razor blade.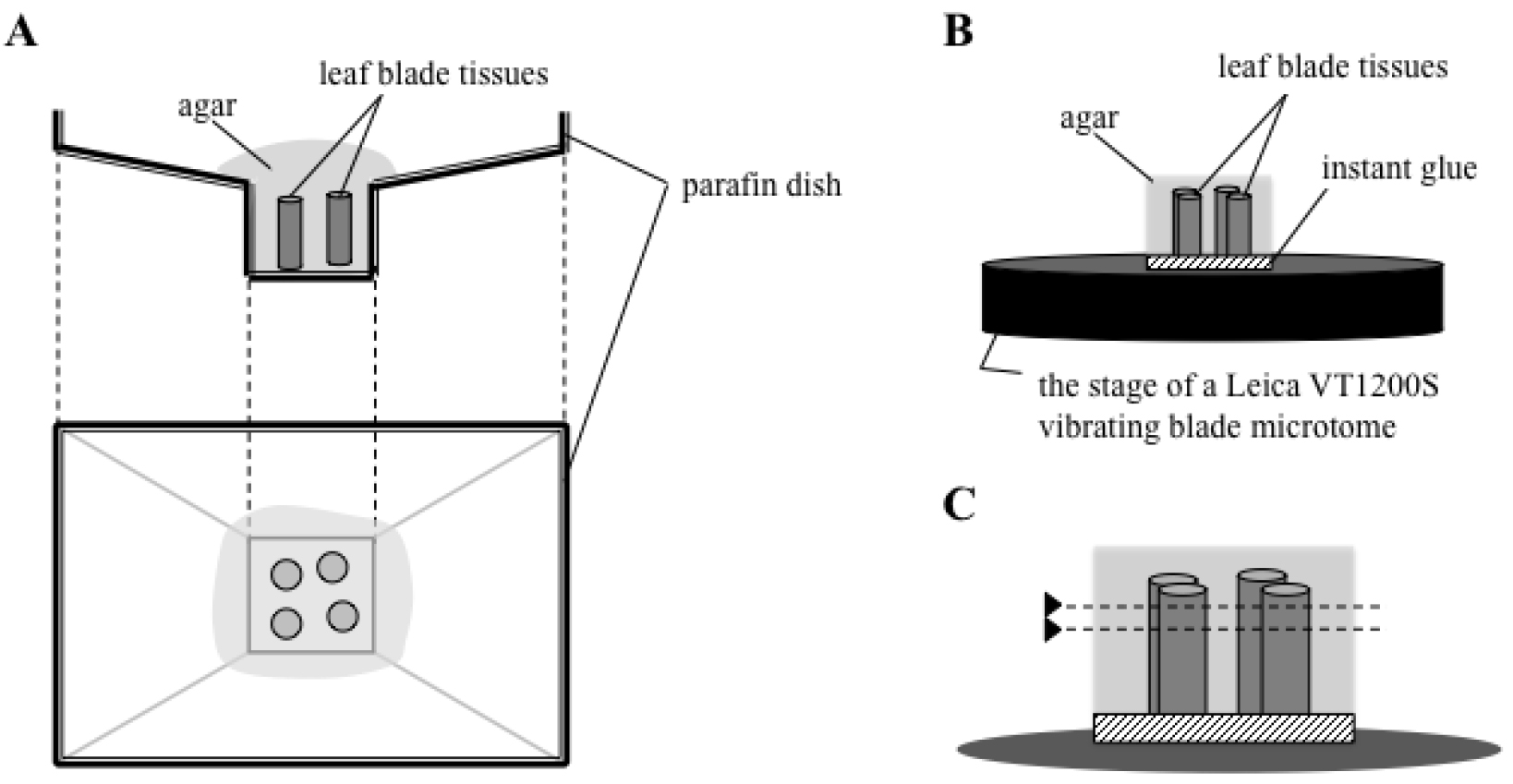
Figure 2. Schematic drawing of section preparation. Rice leaf blade tissues are embedded in agar (A), and the agar block is glued on the stage of a Leica VT1200S vibrating blade microtome (B). The agar-embedded tissue is cut parallel to the surface of the stage (shown as dotted lines in C). - Transfer the sections and incubate them in sequential dehydration treatments with 0.5 ml of 70%, 80%, 90%, and 100% ethanol for 30 min in each solution without shaking.
- The sections are then transferred and incubated for 30 min in each step in increasing concentrations of benzene in ethanol, ranging from 10% up to 100% with 10% stepwise increases, until the solvent has been replaced with benzene.
- The benzene-equilibrated sections are stained with 0.1% crystal violet lactone solution for 10 min to visualize the silicified cells.
- Place the stained sections on a microscope slide and cover it gently with a cover slip. Seal the cover slip with nail polish.
- The sections are observed using an optical microscope and images are recorded using a CCD camera (Figure 2).
Representative data
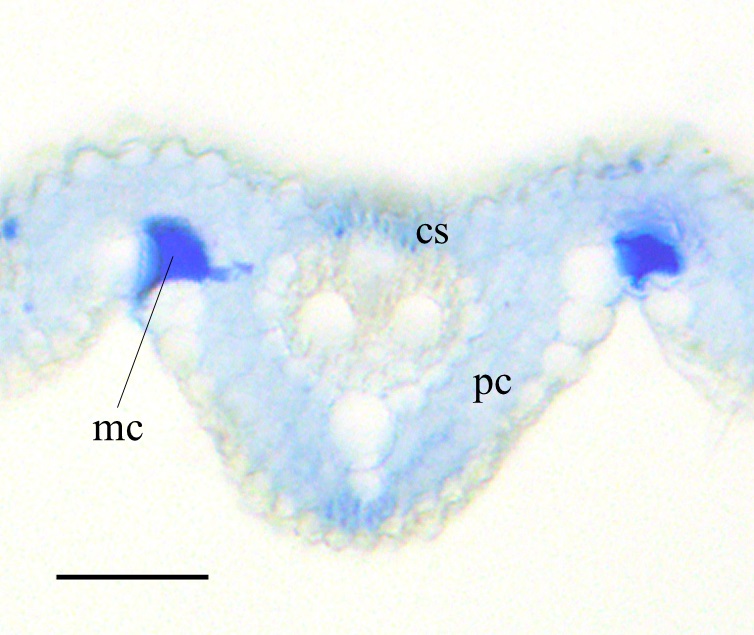
Figure 3. Crystal violet lactone staining of transverse section of the rice leaf blade at foliar age 5.2. cs: cortical sclerenchyma, pc: parenchyma. mc: motor cell. Scale bar = 50 µm
Notes
Rice (Oryza sativa L. cv Nipponbare) plants are grown in a growth chamber at 28 °C under a 15/9 h light/dark cycle (light at 150 μmol-2 s-1). This protocol is performed using the fifth leaf blades of rice plants grown in +Si conditions (Kido et al., 2015).
Recipes
- PBS
Dissolve 10 PBS tablets in distilled water to make a total volume of 1,000 ml - 100% ethanol solution
To prepare the 100% ethanol solution, use 100% bulk ethanol with molecular sieves in the bottom of the bottle - Crystal violet lactone solution
Dissolve crystal violet lactone as a 0.1% solution in benzene
Acknowledgments
This protocol has been adapted or modified from a previous study by Isa et al. (2010). This study was supported by the Japan Society for Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan [a Grant-in-Aid for Scientific Research on Innovative Areas “Plant Cell Wall” (No. 24114001, 24114005) to K.N. and a Grant-in-Aid for Scientific Research (C) (25440124) to R.Y.].
References
- Ichimura, K., Funabiki, A., Aoki, K. and Akiyama, H. (2008). Solid phase adsorption of crystal violet lactone on silica nanoparticles to probe mechanochemical surface modification. Langmuir 24(13): 6470-6479.
- Isa, M., Bai, S., Yokoyama, T., Ma, J.F., Ishibashi, Y., Yuasa, T., Iwaya-Inoue, M. (2010). Silicon enhances growth independent of silica deposition in a low-silica rice mutant, lsi1. Plant Soil 331(1-2): 361-375.
- Kido, N., Yokoyama, R., Yamamoto, T., Furukawa, J., Iwai, H., Satoh, S. and Nishitani, K. (2015). The matrix polysaccharide (1;3,1;4)-beta-D-glucan is involved in silicon-dependent strengthening of rice cell wall. Plant Cell Physiol 56(2): 268-276.
- Yamamoto, T., Nakamura, A., Iwai, H., Ishii, T., Ma, J. F., Yokoyama, R., Nishitani, K., Satoh, S. and Furukawa, J. (2012). Effect of silicon deficiency on secondary cell wall synthesis in rice leaf. J Plant Res 125(6): 771-779.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Yokoyama, R., Kido, N., Yamamoto, T., Furukawa, J., Iwai, H., Satoh, S. and Nishitani, K. (2015). Histochemical Staining of Silica Body in Rice Leaf Blades. Bio-protocol 5(19): e1609. DOI: 10.21769/BioProtoc.1609.
Category
Plant Science > Plant physiology > Ion analysis
Plant Science > Plant cell biology > Cell staining
Biochemistry > Other compound > Ion > Silicon
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









