- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Infection Assay of Cyst Nematodes on Arabidopsis Roots
Published: Vol 5, Iss 18, Sep 20, 2015 DOI: 10.21769/BioProtoc.1596 Views: 12394
Reviewed by: Zhaohui LiuDaniel F. CaddellAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Preparation of Nippostrongylus brasiliensis Larvae for the Study of Host Skin Response
Tiffany Bouchery [...] Nicola L. Harris
Dec 20, 2020 2904 Views

Quantification of Bacterial Loads in Caenorhabditis elegans
Alyssa C. Walker [...] Daniel M. Czyz
Jan 20, 2022 4231 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2760 Views
Abstract
Plant parasitic nematodes are devastating pests on many crops. Juveniles (J2) of cyst nematodes invade the roots to induce a syncytium. This feeding site is their only source of nutrients. Male nematodes leave the roots after the fourth molt to mate with females. The females stay attached to their syncytia throughout their life and produce hundreds of eggs, which are contained in their bodies. When the females die their bodies form the cysts, which protect the eggs. Cysts can survive for many years in the soil until favorable conditions induce hatching of the juveniles.
The beet cyst nematode Heterodera schachtii is a pathogen of sugar beet (Beta vulgaris) but can also complete its life cycle on Arabidopsis roots growing on agar plates under sterile conditions. We present here protocols for a stock culture of H. schachtii and an infection assay on agar plates.
Materials and Reagents
- Heterodera schachtii
- Seeds of Sinapis alba cv Albatros
- Seeds of Arabidopsis thaliana
- Calcium hypochlorite
- HgCl2
- Tween 20
- 70% ethanol
- GAMBORG B5 VITAMIN MIXTURE (Duchefa Biochemie, catalog number: G0415 )
- Daichin agar (Duchefa Biochemie, catalog number: D1004 )
- Saccharose
- KNO3
- MgSO4.7H2O
- Ca(NO3)2.4H2O
- KH2PO4
- FeNaEDTA
- H3BO3
- MnCl2 or MnCl2.4H2O
- CuSO4.5H2O or CuSO4.2H2O
- ZnSO4.7H2O
- CoCl2.6H2O
- H2MoO4 or NaMoO4.2H2O
- NaCl
- ZnCl2
- GELRITETM (Duchefa Biochemie, catalog number: G1101 )
- Plastic Petri dishes 9 cm
- Plastic Petri dishes 14.5 cm
- Aluminum foil
- PVC membrane 100 µm mesh (Buddeberg GmbH, catalog number: 9068289 )
- PVC membrane 15 µm mesh (Buddeberg GmbH, catalog number: 9068280 )
- 50 ml syringes
- Pipettes
- Preparation of water agar plates (see Recipes)
- Preparation of Knop medium (see Recipes)
Equipment
- Nescofilm (or Parafilm)
- Growth chamber at 25 °C with 16 h light/8 h dark
- Laboratory glassware
- Balance
- Stereo microscope
- Inverse microscope with camera
- Clean bench
- Autoclave capable of reaching 120 °C
- Axiovert 200M inverse microscope (ZEISS) with an integrated camera (ZEISS, model: AxioCam MRc5 )
- Funnel for hatching (Figure 1)
Software
- Contour tool of the AxioVision software (ZEISS)
- SPSS 12.0 (SPSS Inc.)
Procedure
- Arabidopsis and mustard seed sterilization:
- Immerse seeds for 10 min in 5% calcium hypochlorite with 0.1% Tween 20, shake a few times.
- Pour off the solution and add 70% ethanol, incubate for 5 min.
- Wash 3 times in sterile water.
- Dry the seeds in a clean bench in a sterile Petri dish.
- Immerse seeds for 10 min in 5% calcium hypochlorite with 0.1% Tween 20, shake a few times.
- Stock culture of H. schachtii:
- Prepare 14.5 cm 0.7% water agar plates.
- Prepare 14.5 cm Knop agar plates.
- Place 20 sterile mustard seeds on 0.7% water agar plate.
- Seal the plates with Nescofilm.
- Store in a dark place at RT for 3-4 days.
- Transfer 3 seedlings onto a 14.5 cm Knop agar plate and seal the plates with Nescofilm.
- Store in a dark place at RT for about 10 days.
- Infect the roots with nematodes (3-4 drops per root); it is convenient to use J2 that are left over from an infection test.
- Keep the plates in a dark place at room temperature until the cysts are needed. It takes approximately 2 months until the cysts can be harvested. It is possible to store the plates for up to 1 year. However, if the agar in the plates becomes too dry it may be more difficult to pick out the cysts.
- Prepare 14.5 cm 0.7% water agar plates.
- Preparation of H. schachtii inoculum:
- Prepare a funnel:
- Assemble the funnel as shown in Figure 1 with a 100 µm sieve.
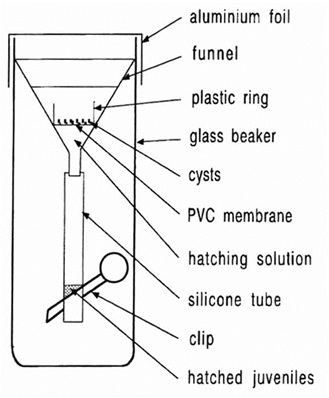
Figure 1. Funnel for hatching. Glass beakers, funnels, silicone tubes, and clips can be bought from local suppliers. - Cover the funnel with aluminum foil.
- Sterilize by autoclaving for 20 min at 120 °C.
- Prepare 15 µm and 100 µm sieves (Figure 2).

Figure 2. 15 µm and 100 µm sieves are needed for sterilization of J2s. Only the 100 µm sieves are shown on this picture. - Cut off 1 cm high rings from a 50 ml syringe.
- Heat one side of the ring and glue it to a piece of PVC membrane (15 µm or 100 µm mesh).
- Sterilize by autoclaving for 20 min at 120 °C.
- Prepare 0.7% gelrite: Add 1.4 g gelrite to 200 ml distilled water, cook until the solution becomes clear.
- Autoclave for 20 min at 120 °C.
- Pour 5 ml into 15 ml tubes (becomes solid at RT).
- Pick cysts from the stock culture on mustard.
- Place the cysts into the 100 µm sieve sitting in a 50 ml glass beaker.
- Soak the cysts in 5% calcium hypochlorite with 0.1% Tween 20 for 10 min.
- Wash 4 times with sterile distilled water.
- Put the sieve with the cysts into the hatching funnel (Figure 1).
- Fill the funnel with 3 mM ZnCl2 (stimulates hatching of the J2).
- Keep the hatching funnel in darkness at RT.
- The hatched larvae move through the 100 µm sieve into the silicone tube and can be collected from the silicone tube (Figure 1) by briefly opening the clip after 3-5 days into a 15 µm sieve.
- Sterilize the J2 for 2 min by putting the 15 µm sieve with the J2 into a beaker containing 0.05% HgCl2.
- Wash four times by transferring the 15 µm sieve with the J2 into a beaker with sterile distilled water.
- Resuspend the J2 in 0.7% gelrite prior to inoculation.
- Adjust the concentration of the inoculum to 20 J2 per drop of gelrite using a pipette by counting the J2 in the drops under a stereo microscope.
- The J2 can be stored for 2-3 days in a fridge at 4 °C without loosing their infectivity.
- Prepare a funnel:
- Arabidopsis cultivation:
- Prepare 9 cm Knop agar plates.
- Grow the Arabidopsis plants:
- Place sterile seeds on Knop agar plate (Figure 3). Use 10 seeds per plate.
Figure 3. Template for Arabidopsis seeds - Incubate the plates for 4 days at 4 °C to ensure uniform germination.
- Place the plates horizontally in the growth chamber for 2 days.
- Tilt the plates to approximately 20 degrees so that the roots will grow to the bottom of the plates between the agar and the Petri dish.
- Prepare 9 cm Knop agar plates.
- Inoculation of Arabidopsis plants:
- Inoculate 12-d-old Arabidopsis roots with 60 J2 per plant by putting 3 drops of gelrite containing the J2 on different parts of the root.
- Incubate horizontally in the dark over night at RT.
- Put the plates back into the light.
- Inoculate 12-d-old Arabidopsis roots with 60 J2 per plant by putting 3 drops of gelrite containing the J2 on different parts of the root.
- Analysis of the nematode infection:
Take pictures of syncytia with female nematodes at 13 dpi.
(We use an Axiovert 200M inverse microscope with an integrated camera) at 14 dpi count the numbers of male and female nematodes (Figure 4).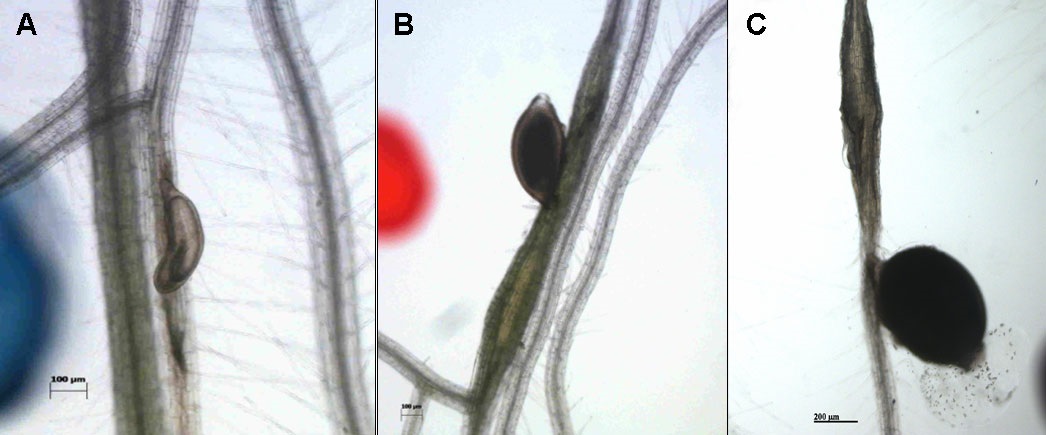
Figure 4. Male (A), female (B) nematode and the cyst (C) - Size measurement of syncytia and cysts (females):
- Select 10 syncytia/cysts at random.
- Outline the cysts and the syncytia [we use the Contour tool of the AxioVision software and the software will calculate the size (Figure 5)].
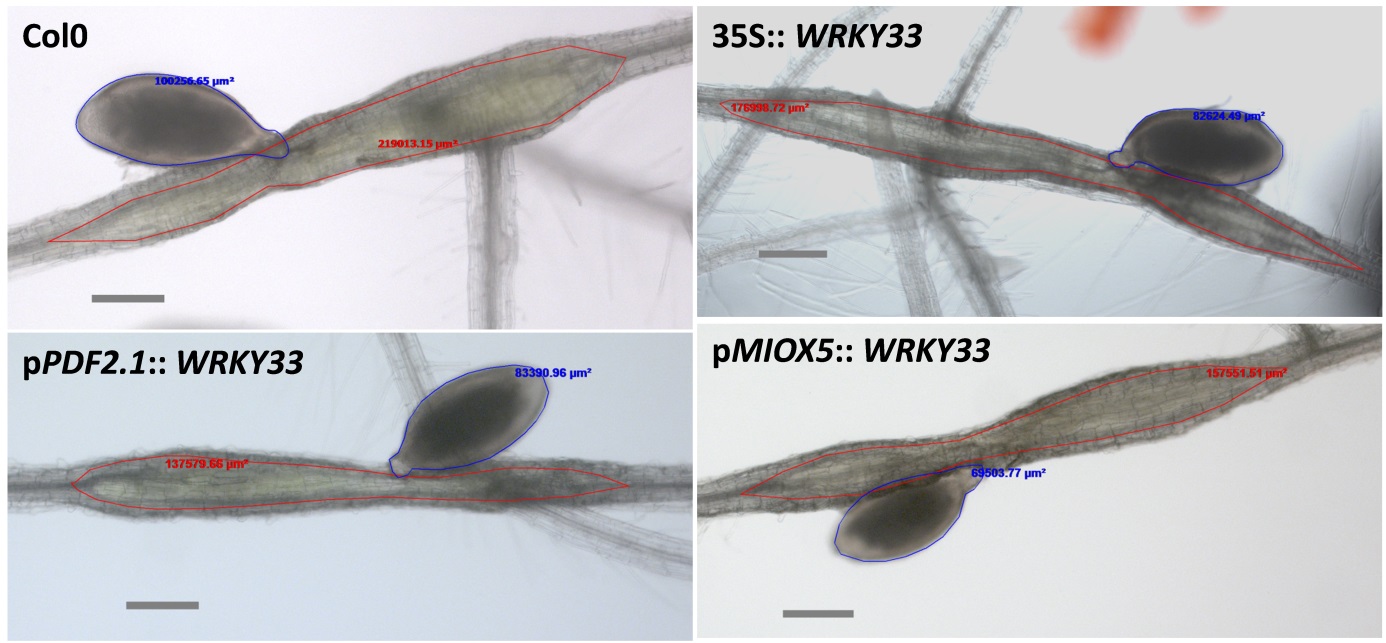
Figure 5. Size measurement of female nematodes and syncytia. Numbers are the sizes calculated by the contour tool of the AxioVision software for nematodes (blue) and syncytia (red). Shown are syncytia induced in transgenic lines overexpressing a WRKY gene and the wild type (Col) [from Ali et al. (2014)] - Analyze the significance of the results using analysis of variance (ANOVA) (P < 0.05) (we use SPSS 12.0).
- Select 10 syncytia/cysts at random.
- Counting the nematodes:
- Use a stereo microscope.
- Invert the plates and use waterproof markers with different colors to mark males and females by placing a dot besides the nematodes.
- Count the dots for males and females.
- Calculate the total number of males and females for each line per plant or root length. From these data calculate the male/female ratio.
- If there is a difference in the growth (size) of control and test (mutants, transgenic lines) lines at the time of inoculation classify the lines on the Petri dishes according to Figure 6.
- Analyze the significance of the results using analysis of variance (ANOVA) (P < 0.05) (we use SPSS 12.0).
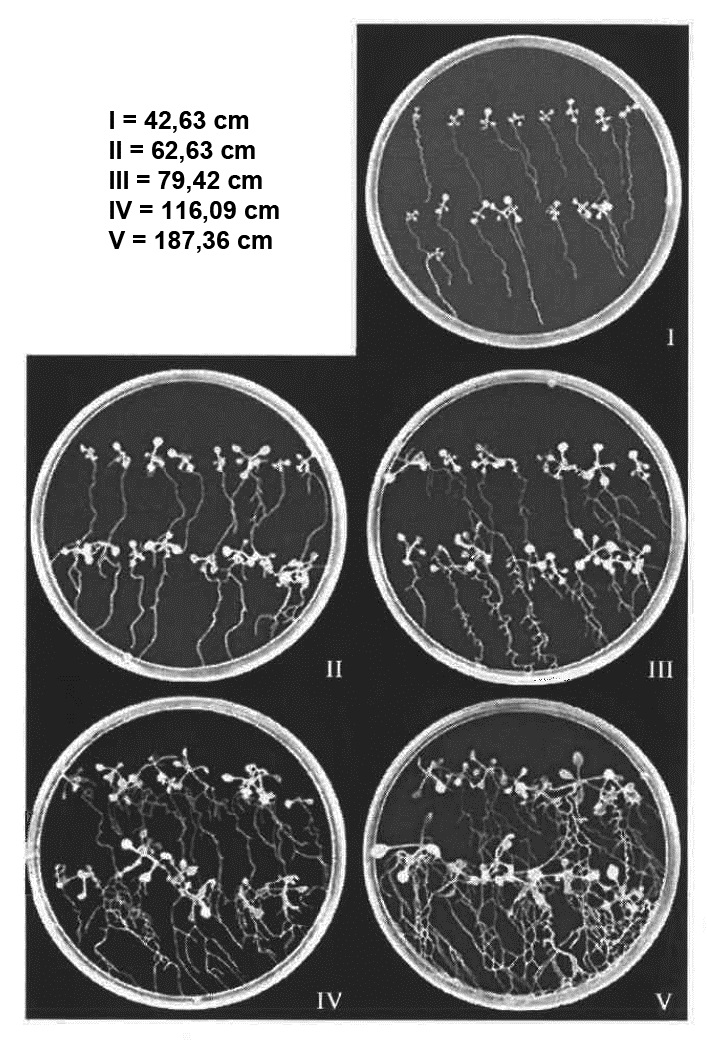
Figure 6. Growth classes of Arabidopsis plants. This figure can be used to classify the Arabidopsis lines if there are differences in plant growth to adjust the number of nematodes [from Jürgensen (2001)].
- Use a stereo microscope.
Notes
- It is known that in comparison to male nematodes more female nematodes can develop under optimal nutritional and environmental conditions. Therefore the female/male ratio (F/M) is a meaningful indicator for the developmental conditions provided by the host plant (Triantaphyllou, 1973).
- It was found that growth of Arabidopsis plants under sterile conditions for infection with H. schachtii was best on 0.2x concentrated Knop medium (Sijmons et al., 1992) prepared with Daichin agar. Other agar types may also work but have to be tested.
- There is seasonal variation in growth and hatching of the nematodes. During the summer months the hatching and infection rate is significantly higher than during the wintertime.
- Each test should include a control to adjust for differences in inoculum.
- We do three replicates with 40 plants per line (4 Petri dishes).
- Growth of plants and nematodes is under sterile conditions. Also the preparation of the inoculum has to be done under sterile conditions.
Recipes
- Preparation of water agar plates
- Combine 7 g Daichin agar and 1 L distilled water and autoclave for 20 min at 120 °C
- Pour into 14.5 cm Petri dishes
- Combine 7 g Daichin agar and 1 L distilled water and autoclave for 20 min at 120 °C
- Preparation of Knop medium
- Prepare the following stock solutions for Knop medium (per liter)
Stock solution Chemical g per liter Alternative chem. g per liter Stock solution I KNO3 121.32 g MgSO4.7H2O 19.71 g Stock solution II Ca(NO3)2.4H2O 120 g Stock solution III KH2PO4 27.22 g Stock solution IV FeNaEDTA 7.34 g Stock solution V H3BO3 2,86 g MnCl2 1.81 g MnCl2.4H2O 2.85 g CuSO4.5H2O 0.073 g CuSO4.2H2O 0.05 g ZnSO4.7H2O 0.36 g CoCl2.6H2O 0.03 g H2MoO4 0.052 g NaMoO4.2H2O 0.0775 g NaCl 2 g - Prepare 1 L, 0.2x Knop agar
Saccharose 20 g Daichin agar 8 g B5 Vitamins 1 ml Stock solution I 2 ml Stock solution II 2 ml Stock solution III 2 ml Stock solution IV 0.4 ml Stock solution V 0.2 ml - Add deionized water to 1 L
- Adjust the pH to 6.4 with KOH solution
- Autoclave for 20 min at 120 °C and pour plates the same day
- Prepare the following stock solutions for Knop medium (per liter)
Acknowledgments
This work was supported by FWF Austrian Science Fund grants P16296-B06, P16897-B06 and P21067-B12.
References
- Ali, M. A., Wieczorek, K., Kreil, D. P. and Bohlmann, H. (2014). The beet cyst nematode Heterodera schachtii modulates the expression of WRKY transcription factors in syncytia to favour its development in Arabidopsis roots. PLoS One 9(7): e102360.
- Jürgensen, K (2001). Untersuchungen zum Assimilat- und Wassertransfer in der Interaktion zwischen Arabidopsis thaliana und Heterodera schachtii. Dissertation Kiel, Germany: Christian-Albrechts Universität.
- Sijmons, P. C., Grundler, F. M. W., von Mende, N., Burrows, P. R. and Wyss, U. (1991). Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J 1: 245-254.
- Triantaphyllou, A. C. (1973). Gametogenesis and reproduction of Meloidogyne graminis and M. ottersoni (Nematoda: Heteroderidae). J Nematol 5(2): 84-87.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Bohlmann, H. and Wieczorek, K. (2015). Infection Assay of Cyst Nematodes on Arabidopsis Roots. Bio-protocol 5(18): e1596. DOI: 10.21769/BioProtoc.1596.
Category
Microbiology > Microbe-host interactions > In vitro model
Microbiology > Microbe-host interactions > Nematode
Plant Science > Plant immunity > Disease bioassay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










