- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of Chlorophyll a and Carotenoids Concentration in Cyanobacteria
Published: Vol 5, Iss 9, May 5, 2015 DOI: 10.21769/BioProtoc.1467 Views: 36813
Reviewed by: Seda EkiciFanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

β-lactamase (Bla) Reporter-based System to Study Flagellar Type 3 Secretion in Salmonella
Fabienne F. V. Chevance and Kelly T. Hughes
Jun 20, 2023 1764 Views

Determination of Poly(3-hydroxybutyrate) Content in Cyanobacterium Synechocystis sp. PCC 6803 Using Acid Hydrolysis Followed by High-performance Liquid Chromatography
Janine Kaewbai-ngam [...] Tanakarn Monshupanee
Aug 20, 2023 1808 Views

An HPLC-based Assay to Study the Activity of Cyclic Diadenosine Monophosphate (C-di-AMP) Synthase DisA from Mycobacterium smegmatis
Avisek Mahapa [...] Dipankar Chatterji
Dec 20, 2024 1779 Views
Abstract
This is a protocol for precise measurement of chlorophyll a and total carotenoid concentrations in cyanobacteria cells. Cellular chlorophyll concentration is one of the central physiological parameters, routinely followed in many research areas ranging from stress physiology to biotechnology. Carotenoids concentration is often related to cellular stress level; combined pigments assessment provides useful insight into cellular physiological state. The current protocol was established to minimize time and equipment requirements for the routine pigments analysis. It is important to note that this protocol is suitable only for cyanobacteria containing chlorophyll a, and is not designed for species containing other chlorophyll molecules.
Keywords: PigmentsMaterials and Reagents
- Cyanobacteria culture (Note 1)
- Methanol ≥99.9% (GC) (Sigma-Aldrich)
Equipment
- Eppendorf safe-lock tubes (1.5 ml)
- Centrifuge with relative centrifugal force (RCF) of 15,000 x g and cooling option to +4 °C (Sigma-Aldrich, model: 1-16 K )
- Pipette 100 µl -1,000 µl + pipette tips (RAININ, Mettler-Toledo)
- Fridge (+4 °C)
- Spectrophotometer with slit width 1 nm (Shimadzu, model: UV-2600 )
- Spectrophotometric plastic or glass VIS/UV-VIS semi-micro 0.75-1.5 ml cuvettes
- Mixing device (Silamat S6, Ivoclar Vivadent) or vortex (IKA MS3 digital, IKA®)
- Aluminum foil
- Holder for Eppendorf tubes
Procedure
- Work under modest irradiance [up to 5 µmol (photons) m-2 s-1 of white light or 10 µmol (photons) m-2 s-1 of green light] in order to prevent degradation of extracted pigments.
- Harvest 1 ml of cyanobacterial culture suspension (Note 2).
- Centrifuge cells at 15,000 x g at laboratory temperature for 7 min and thoroughly discard supernatant. If necessary repeat the centrifugation (Note 3).
- Add 1 ml of methanol, precooled to +4 °C.
- Homogenize the sample by mixing (Silamat S6, 2 sec), vortexing (2,000 rpm, 4 sec), or by gentle pipetting up and down.
- Cover the samples with aluminum foil. Incubate at +4 °C for 20 min in order to extract the pigments from the cells (Note 4).
- Centrifuge at 15,000 x g, +4 °C for 7 min and visually check pellet; it should be ranging between bluish and purple (Figure 1) with no green color. If the pellet is green, repeat steps 5-6.
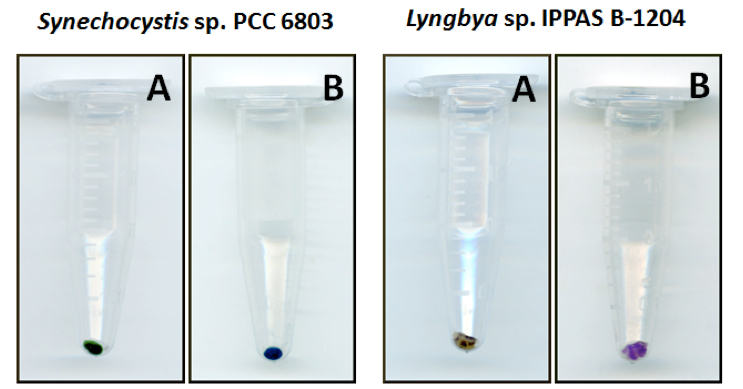
Figure 1. Colors of cyanobacteria pellets before addition of methanol (A) and after chlorophyll a and carotenoids extraction (B). The color of pellets after the methanol extraction will be ranging from bluish (Synechocystis sp. PCC 6803) to purple (Lyngbya sp. IPPAS B-1204), depending on particular combination of phycobiliproteins. - Calibrate spectrophotometer using methanol as blank.
- Measure pigments concentration by spectrophotometer with slit width 1 nm.
- Measure absorbance of sample and blank at 470 nm, 665 nm and 720 nm (Note 5).
- Calculate concentration of chlorophyll a content according to equations:
Chla [µg/ml] = 12.9447 (A665 − A720) (Ritchie, 2006)
Chla [µM] = 14.4892 (A665 − A720); for Chla molar mass = 893.4890 g/mol
Carotenoids [µg/ml] = [1,000 (A470 − A720) − 2.86 (Chla [µg/ml])] / 221 (Wellburn, 1994).
- Measure absorbance of sample and blank at 470 nm, 665 nm and 720 nm (Note 5).
- Perform the analysis at least in triplicates as necessary for calculations of averages and standard deviations from each pigments assessment.
Representative data
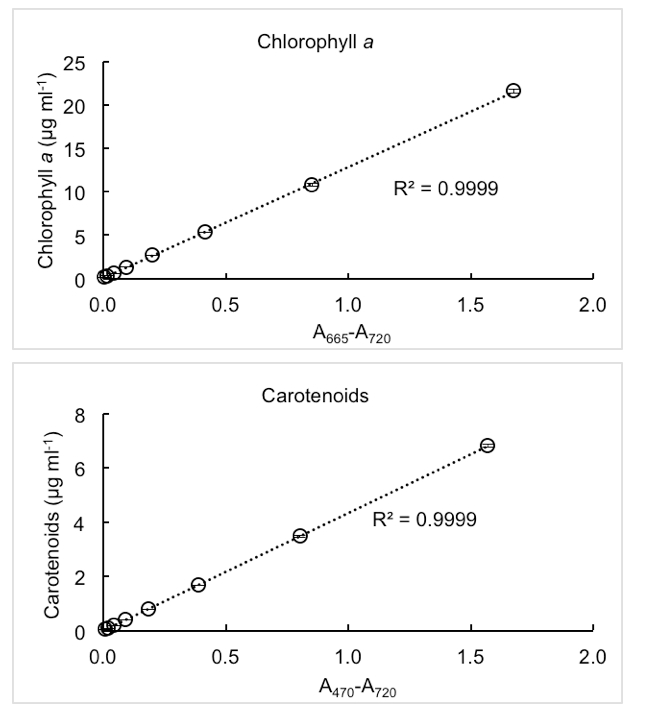
Figure 2. Representative measurements of chlorophyll a and carotenoids concentrations in cell culture of cyanobacterium Synechocystis sp. PCC 6803. Dense cyanobacteria culture (chlorophyll a: 22 µg/ml, carotenoids: 7 µg/ml) was gradually diluted by half up to pigments concentration 0.1 µg/ml. Each measurement was performed in triplicates; the error bars represent standard deviations.
Notes
- The authors are not aware of any restrictions of this protocol usage for cyanobacterial strains containing only chlorophyll a. The protocol was successfully applied to Cyanothece sp. ATCC 51142, Synechococcus elongatus sp. PCC 7942, Cyanobacterium sp. IPPAS B-1200, Synechocystis sp. PCC 6803, Arthrospira platensis IPPAS B-256, Anabaena sphaerica IPPAS B-404, Chroococcus sp. IPPAS B-1203, Lyngbya sp. IPPAS B-1204, Aphanocapsa sp. IPPAS B-1205, Anabaena sp. IPPAS B-1206.
- The amount of cell suspension required for analysis can vary with the culture density. With very diluted cultures it is recommended to harvest bigger culture volume; for cultures with chlorophyll a density around 10 ng(Chl) mlculture-1 or lower even up to 5 ml. On the contrary, for very dense cultures lower sample volume is recommended – with high cellular densities, some pigments can remain in cells after extraction.
- If some supernatant remains in the tube after the first centrifugation, the extraction will not take place in pure methanol and the pigment concentrations will not be measured properly.
- The pigment extraction time can be prolonged up to 2 h with no significant pigment degradation.
- The final absorbance at each wavelength should be in linear absorbance range. For spectrophotometer UV-2600 (Shimadzu) this linear absorbance range is 0.01 - 2.5. If necessary, dilute the sample with methanol to fit in the spectrophotometer linear absorbance range.
- In case of using different volumes of cyanobacteria samples and/or methanol than 1 ml, the final pigment concentration should be calculated according to following equation:
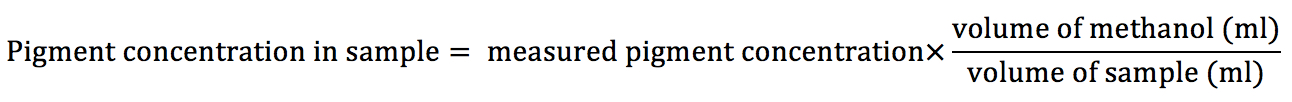
Acknowledgments
The protocol was adopted from publication “On the dynamics and constraints of batch culture growth of the cyanobacterium Cyanothece sp. ATCC 51142” (Sinetova et al., 2012). T. Z. and J. C. were supported by the MEYS CR within CzechGlobe Centre, reg. no. CZ.1.05/1.1.00/02.0073 484, the National Sustainability Program I (NPU I), grant number LO1415 and by EC OP project, reg. no. CZ.1.07/2.3.00/20.0256. S. M. A. was supported by grant from Russian Scientific Foundation no. 14-24-00020).
References
- Balizs, G., Benesch-Girke, L., Borner, S. and Hewitt, S. A. (1994). Comparison of the determination of four sulphonamides and their N4-acetyl metabolites in swine muscle tissue using liquid chromatography with ultraviolet and mass spectral detection. J Chromatogr B Biomed Appl 661(1): 75-84.
- Ritchie, R. J. (2006). Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89(1): 27-41.
- Sinetova, M. A., Červený, J., Zavřel, T. and Nedbal, L. (2012). On the dynamics and constraints of batch culture growth of the cyanobacterium Cyanothece sp. ATCC 51142. J Biotechnol 162(1): 148-155.
- Wellburn, A. R. (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zavřel, T., Sinetova, M. A. and Červený, J. (2015). Measurement of Chlorophyll a and Carotenoids Concentration in Cyanobacteria. Bio-protocol 5(9): e1467. DOI: 10.21769/BioProtoc.1467.
Category
Microbiology > Microbial biochemistry > Other compound
Biochemistry > Other compound > Chlorophyll
Biochemistry > Other compound > Carotenoid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









