- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Chitin Binding Assay
Published: Vol 5, Iss 4, Feb 20, 2015 DOI: 10.21769/BioProtoc.1401 Views: 13022
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Analytical Gel Filtration for Probing Heavy Metal Transfer between Proteins
Steffen Lorenz Drees and Mathias Lübben
Aug 5, 2016 11103 Views

Hydrogen Deuterium Exchange Mass Spectrometry of Oxygen Sensitive Proteins
Luke Berry [...] Brian Bothner
Mar 20, 2018 10251 Views

Binding Affinity Quantifications of the Bacteriophage Mu DNA Modification Protein Mom Using Microscale Thermophoresis (MST)
Shubha Udupa [...] Shweta Karambelkar
Jul 20, 2022 3698 Views
Abstract
Chitin is polymer of N-acetylglucosamine (GlcNAc) found in the exoskeleton of arthropods and the fungal cell wall. GlcNAc is also implicated in bacterial development, adherence, and signal transduction but can also be used as a carbon source. In vitro chitin binding assay is performed to determine the affinity of a purified protein to the chitin molecule. The principle is based on the co-sedimentation of chitin-binding proteins together with chitin-coated beads.
Keywords: ChitinMaterials and Reagents
- Purified protein with chitin binding affinity
Note: We used histidine-tagged chitin binding protein CbpD. The protein was purified by affinity chromatography onto a 5-ml HisTrap nickel column (Pharmacia) on an Äkta system (Amersham Biosciences). The complete purification protocol is described in details in Cadoret et al. (2014).
- Chitin beads (New England Biolabs, catalog number: S6651 )
- Tris Base
- EDTA
- NaCl
- Tween 100
- Freshly made solution of chitin binding buffer (see Recipes)
Equipment
- Laboratory vortex adapted to Eppendorf tubes
- Laboratory rotating wheel adapted to Eppendorf tubes
- Cold room or refrigerated incubator (4 °C)
Procedure
- Wash the chitin beads as follows
- Vortex the chitin beads before pipetting.
- Wash the desired amount of beads solution (100 µl per experiment) twice with 5 volumes of chitin binding buffer. This washing step is performed by gravity flow by leaving the sample 5 min at room temperature.
- Vortex the chitin beads before pipetting.
- Binding assay
- Prepare a 200 µl solution of chitin-binding buffer containing the purified CbpD protein at 60 µg/ml (total fraction). For that, 1.5 µl of purified CbpD at 8 mg/ml in 50 mM Tris pH 8, 300 mM NaCl, 250 mM imidazole was mixed with 198. 5 µl of chitin binding buffer.
- Add 100 µl of pre-washed chitin beads in chitin-binding buffer to the purified protein solution.
- Incubate the sample on a laboratory rotating wheel for 90 min at 4 °C.
- After gravity flow separation, collect the supernatant (unbound fraction).
- Wash the beads (bound fraction) twice with 200 µl of chitin binding buffer by gravity flow.
- Analyse 15 μl of Total (equivalent to 0.9 μg), Unbound and Bound fractions by SDS-PAGE followed by Coomassie blue staining and/or immunoblotting (see Figure 1).
- Prepare a 200 µl solution of chitin-binding buffer containing the purified CbpD protein at 60 µg/ml (total fraction). For that, 1.5 µl of purified CbpD at 8 mg/ml in 50 mM Tris pH 8, 300 mM NaCl, 250 mM imidazole was mixed with 198. 5 µl of chitin binding buffer.
Representative data
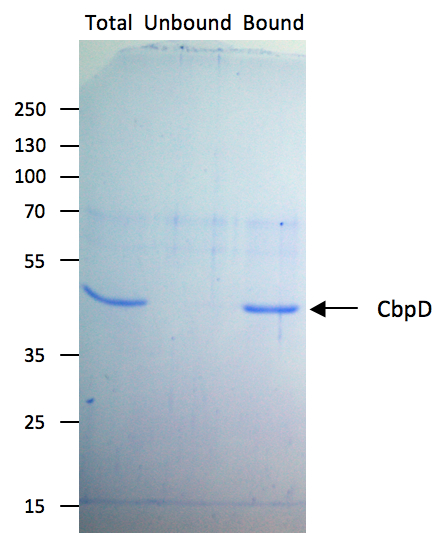
Figure 1. Chitin-binding affinity of CbpD. To test the chitin-binding affinity of CbpD, a solution of purified CbpD at 200 μg/ml in chitin binding buffer (Total) was incubated with chitin beads according to the present protocol. 15 μl samples of Total, Unbound and Bound fractions were analyzed by SDS-PAGE followed by Coomassie blue staining. Molecular mass markers (in kDa) are indicated on the left.
Notes
- The chitin binding buffer and the protein solution in chitin binding buffer must be freshly prepared.
- A negative control can consist of the same experiment where the chitin beads solution is replaced by chitin binding buffer.
- Additionally, purified proteins without chitin binding affinity such as Elastase (LasB) and exotoxin A (ToxA) can be used as negative control [see Figure 5B in Cadoret et al. (2014)].
Recipes
- Freshly made solution of chitin binding buffer
Chitin binding buffer:
50 mM Tris HCl (pH 8)
1 mM EDTA (pH 8)
500 mM NaCl
0.1% Tween 100
The chitin binding buffer is prepared from stock solutions:
Tris HCl 1 M (pH 8)
EDTA 0.5 M (pH 8)
NaCl 5 M
Acknowledgments
This protocol adapted from Shutinoski et al. (2010) was used in Cadoret et al. (2014). The work is funded by a Ph.D. grant from the French government.
References
- Cadoret, F., Ball, G., Douzi, B. and Voulhoux, R. (2014). Txc, a new type II secretion system of Pseudomonas aeruginosa strain PA7, is regulated by the TtsS/TtsR two-component system and directs specific secretion of the CbpE chitin-binding protein. J Bacteriol 196(13): 2376-2386.
- Shutinoski, B., Schmidt, M. A. and Heusipp, G. (2010). Transcriptional regulation of the Yts1 type II secretion system of Yersinia enterocolitica and identification of secretion substrates. Mol Microbiol 75(3): 676-691.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ball, G., Cadoret, F. and Voulhoux, R. (2015). In vitro Chitin Binding Assay. Bio-protocol 5(4): e1401. DOI: 10.21769/BioProtoc.1401.
Category
Biochemistry > Carbohydrate > Polysaccharide
Biochemistry > Protein > Interaction > Protein-ligand interaction
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









