- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Pain Assessment Using the Rat and Mouse Formalin Tests
Published: Vol 4, Iss 21, Nov 5, 2014 DOI: 10.21769/BioProtoc.1288 Views: 28086
Reviewed by: Soyun KimAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1295 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1810 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1470 Views
Abstract
The formalin test was originally developed by Dubuisson and Dennis (1977), and has since been extensively used to assess pain-related responses. Rats and mice are the most frequently used animal models, though other species including cats, rabbits, guinea pigs, Octodon degus, domestic fowls, crocodiles, tortoises, toads and primates have also been employed. The injection of formalin into the skin of rodent hindpaws to cause spontaneous pain-related flinch behaviors is the most commonly used procedure. The resulting nociceptive response can be divided into two phases differing in timing, duration and underlying mechanisms, and is responsive to many classes of analgesic drugs (Coderre et al., 1990; Hunskaar and Hole, 1987; Rosland et al., 1990; Taylor et al., 1995). The behavioral and electrophysiological responses to formalin consist of an acute phase (Phase I) of a short-lasting response, which is believed to reflect the activity of C-fiber afferent nociceptors. After a short quiescent period, the acute phase is followed by a continuous prolonged response (Phase II), which is believed to be due to central sensitization of the spinal dorsal horn neurons as a result of the initial barrage of input from C-fiber nociceptive afferents during the early phase (Coderre et al., 1993; Dickenson and Sullivan, 1987; Raboisson et al., 1995; Shibata et al., 1989). In this respect, the formalin test has been regarded as a more satisfactory model of pain than tests producing phasic pain like the hot-plate and tail-flick tests (Abbott et al., 1981). Here, we describe the procedures for generating an efficient and reproducible formalin test in rats and mice.
Keywords: Formalin testMaterials and Reagents
- Eight-week-old Wistar (or other strain) rats (body weight 200-250 g)
- Eight-week-old Swiss mice or a variety of transgenic/knockout mice (body weight 20-25 g)
- Sterile normal saline (0.9% NaCl solution) (Thermo Fisher Scientific, catalog number: BR0053G ) or other vehicles for the test articles
- Formaldehyde solution (37 wt.% in H2O) (Sigma-Aldrich, catalog number: 252549 )
- 5% formalin in saline (see Recipes)
Equipment
- Body weight scales for rats (range 0.01-2,000 g) (Shenzhen Amput Electronic Technology Co., catalog number: APTP452) and mice (range 0.01-300 g) (Shenzhen Amput Electronic Technology Co., catalog number: APTP457B)
- Permanent black marker (Thermo Fisher Scientific, catalog number: S02727)
- Individual transparent polycarbonate observation chambers with hinged lids (the formalin test chamber: 30 x 20 x 15 cm for rats and 15 x 15 x 15 cm for mice)
- Mirrors (two or more mirrors are recommended to cover the experimental area)
- Thick cotton bag with an open end
- 0.5-ml syringes with 28-G needles (Thermo Fisher Scientific, catalog number: 22-004-271) or 50-μl Hamilton syringes with 30-G needles (Hamilton Company, catalog number: 80508)
- Hand-held counters
- Timers (two timers are recommended)

Figure 1. Example preparation for the rat formalin test
Software
- Prism software (version 5.01, GraphPad Software Inc.)
Procedure
- Remove the animal from the home cage and record its body weight. Mark the hindpaw that will be injected with formalin with a permanent black marker.
- Place the marked animal into the formalin test chamber that will be used during testing. Place the mirrors behind and beside the container to ensure that the hindpaws can be seen from all angles. It is recommended to have two mirrors used, one is behind the animals and the other one is beside or below the animals. Allow animals to acclimate for 15 to 30 min.
- Prepare the 5% formalin solution in saline. For rats, load 50 μl of 5% formalin in a 0.5-ml syringes equipped with a 28-G needle; for mice, load 10 μl of 5% formalin in a 50-μl Hamilton syringes equipped with a 30-G needle. Load one formalin syringe for each animal to be tested.
- Remove the animal from its container and place it in the thick cotton bag with the marked hindpaw outside in the open end (the thick cotton bag is used to restrain the animal movement when doing the formalin injection). Inject the 5% formalin solution into the dorsal surface of the paw by placing the needle above the toes and below the ankle and inserting it beneath the surface of the skin.
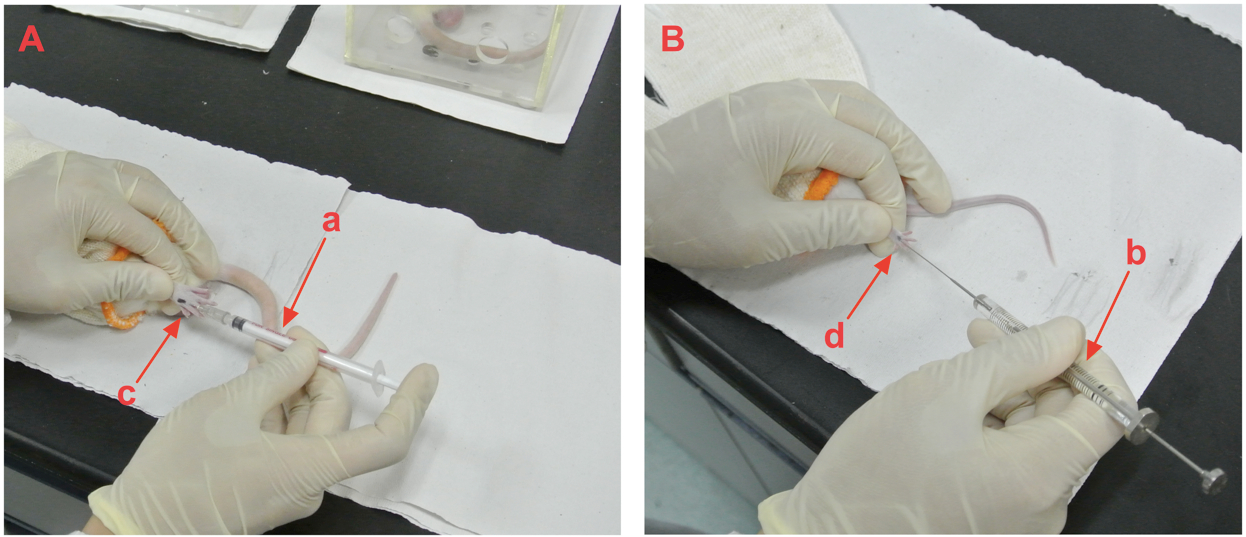
Figure 2. Example pictures showing the formalin injection on the rat A and mouse B. a and b showing the syringes used for rat and mouse; c and d showing the injection sites on the rat and mouse, respectively. - Immediately return the injected animal to its container, and start the timer to mark the beginning of Phase I. Flinching of the paw is the most consistent behavioral response in rats after formalin injection. In contrast, mice primarily show paw-licking behaviors.
- Repeat formalin injections for the remaining animals. Make every effort to complete injections as quickly as possible. Generally, one investigator can monitor six rats or two mice during a single experiment lot.
- For rats, observe the first rat for 1 min and count the number of flinches of the injected hindpaw. Repeat for the remaining five rats. When the sixth rat has been observed, repeat the sequence starting with the first rat in 1-min epochs at 10-min intervals beginning immediately after formalin injection and ending 90 min later. The Phase I response occurs during the first 1 min, and the Phase II response occurs from 20-90 min after formalin injection.
- When quantifying flinches, a brisk elevation of the foot is scored as a single flinch. At times, the animal may exhibit several fast flinches that are impossible to quantify. These episodes are typically scored as one count.
- For mice, observe for 5 min and record the time spent licking during this period. Inject the next mouse 5 min after the first one and record the licking time. Then observe from 20-40 min after formalin injection.
- Like in rats, Phase I and II occur 0-5 min and 20-40 min after the formalin injection, respectively.
- It is recommended that prior to running tests with treatments of interest, investigators inject a few control animals to familiarize themselves with the different flinching behaviors that occur during Phase I and II.
- Like in rats, Phase I and II occur 0-5 min and 20-40 min after the formalin injection, respectively.
- Refer to Video 1 for the formalin injection procedures and formalin-induced flinch behaviors in the rat and mouse.
Video 1. The formalin injection procedures and formalin-induced flinch behaviors in the rat and mouse
- For rats, observe the first rat for 1 min and count the number of flinches of the injected hindpaw. Repeat for the remaining five rats. When the sixth rat has been observed, repeat the sequence starting with the first rat in 1-min epochs at 10-min intervals beginning immediately after formalin injection and ending 90 min later. The Phase I response occurs during the first 1 min, and the Phase II response occurs from 20-90 min after formalin injection.
- Record the total number of flinches in rats or the total time licking in mice for each animal during Phase I and II. Repeat steps 1 to 8 until the desired number of animals (e.g., n = 6-8) in each treatment group have been studied. With each run, it is recommended to have as many treatments as possible presented in parallel.
- Perform data analysis to determine statistical significance. For rats, data may be presented as total flinches per minute for Phase I and II (Figure 3A). The area under curve (AUC) over 10-90 minutes may be calculated for Phase II using the Prism software (Gong et al., 2014a; Gong et al., 2011; Gong et al., 2014b; Gong et al., 2014c; Zhang et al., 2013). For mice, data may be presented as the total licking time during 0-5 min for Phase I (the acute phase) and 20-40 min for Phase II (the tonic phase) (Figure 3B) (Gong et al., 2012; Lu et al., 2012; Zhu et al., 2014).
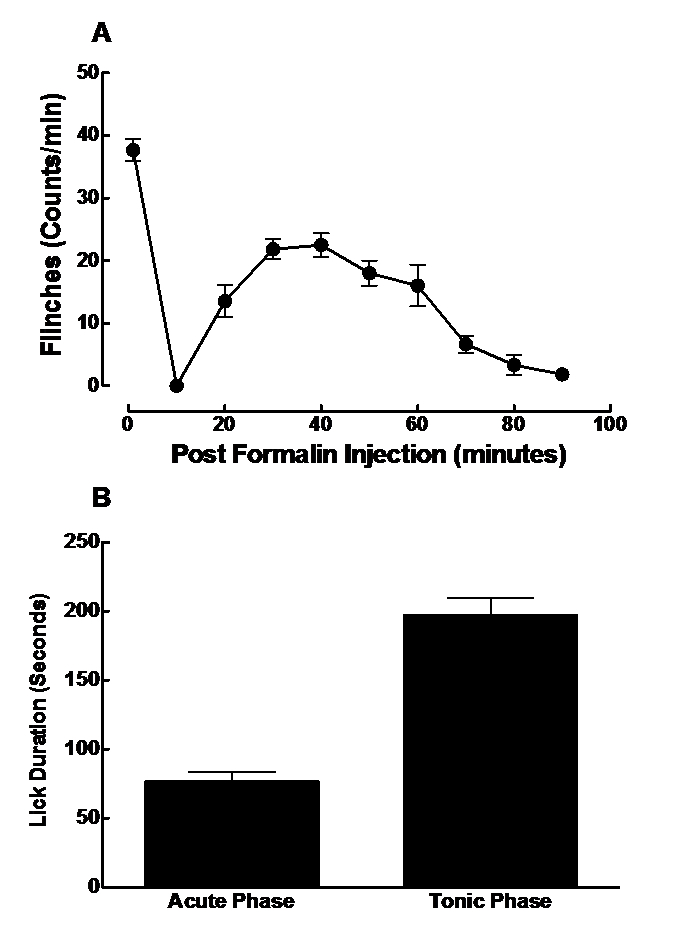
Figure 3. Typical data of the formalin tests from rats A and mice B. In the rat formalin test, the nociceptive behavior was quantified by counting the number of the formalin-injected paw flinches in 1-min epochs on rats. Subcutaneous injection of formalin in rats produced a characteristic bi-phasic flinching response consisting of an initial, rapidly decaying acute phase (Phase I, within 10 minutes after formalin injection) followed by a slowly rising and long-lived (10-90 min) tonic phase (Phase II). In the mouse, the nociceptive behavior was quantified by counting the total licking time. Subcutaneous injection of formalin in mice produced a characteristic bi-phasic licking response consisting of an initial, rapidly decaying acute phase (Phase I, within 5 minutes after formalin injection) followed by a slowly rising and long-lived (20-40 min) tonic phase (Phase II). Data are presented as means ± SEM (n = 6 in each group). More representative figures have been published elsewhere (Gong et al., 2014a; Gong et al., 2011; Gong et al., 2014b; Gong et al., 2012; Gong et al., 2014c; Lu et al., 2012; Zhang et al., 2013; Zhu et al., 2014).
Recipes
- 5% formalin in saline
50 μl formaldehyde solution (37 wt. % in H2O)
950 μl sterile normal saline (0.9% NaCl solution)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81374000), the Doctoral Mentor Fund (No. 20110073110062) from the Ministry of Education of China, and Predoctoral Fellowship to N.G. from the Ministry of Education of China, Shanghai Jiao Tong University, and SJTU School of Pharmacy.
References
- Abbott, F. V., Franklin, K. B., Ludwick, R. J. and Melzack, R. (1981). Apparent lack of tolerance in the formalin test suggests different mechanisms for morphine analgesia in different types of pain. Pharmacol Biochem Behav 15(4): 637-640.
- Coderre, T. J., Katz, J., Vaccarino, A. L. and Melzack, R. (1993). Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain 52(3): 259-285.
- Coderre, T. J., Vaccarino, A. L. and Melzack, R. (1990). Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res 535(1): 155-158.
- Dickenson, A. H. and Sullivan, A. F. (1987). Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neurosci Lett 83(1-2): 207-211.
- Dubuisson, D. and Dennis, S. G. (1977). The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4(2): 161-174.
- Gong, N., Fan, H., Ma, A. N., Xiao, Q. and Wang, Y. X. (2014a). Geniposide and its iridoid analogs exhibit antinociception by acting at the spinal GLP-1 receptors. Neuropharmacology 84: 31-45.
- Gong, N., Gao, Z. Y., Wang, Y. C., Li, X. Y., Huang, J. L., Hashimoto, K. and Wang, Y. X. (2011). A series of D-amino acid oxidase inhibitors specifically prevents and reverses formalin-induced tonic pain in rats. J Pharmacol Exp Ther 336(1): 282-293.
- Gong, N., Li, X. Y., Xiao, Q. and Wang, Y. X. (2014b). Identification of a novel spinal dorsal horn astroglial D-amino acid oxidase-hydrogen peroxide pathway involved in morphine antinociceptive tolerance. Anesthesiology 120(4): 962-975.
- Gong, N., Wang, Y. C., Wang, H. L., Ma, A. N., Hashimoto, K. and Wang, Y. X. (2012). Interactions of the potent D-amino acid oxidase inhibitor CBIO with morphine in pain and tolerance to analgesia. Neuropharmacology 63(3): 460-468.
- Gong, N., Xiao, Q., Zhu, B., Zhang, C. Y., Wang, Y. C., Fan, H., Ma, A. N. and Wang, Y. X. (2014b). Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J Neurosci 34(15): 5322-5334.
- Hunskaar, S. and Hole, K. (1987). The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30(1): 103-114.
- Lu, J. M., Gong, N., Wang, Y. C. and Wang, Y. X. (2012). D-Amino acid oxidase-mediated increase in spinal hydrogen peroxide is mainly responsible for formalin-induced tonic pain. Br J Pharmacol 165(6): 1941-1955.
- Raboisson, P., Dallel, R., Clavelou, P., Sessle, B. J. and Woda, A. (1995). Effects of subcutaneous formalin on the activity of trigeminal brain stem nociceptive neurones in the rat. J Neurophysiol 73(2): 496-505.
- Rosland, J. H., Tjolsen, A., Maehle, B. and Hole, K. (1990). The formalin test in mice: effect of formalin concentration. Pain 42(2): 235-242.
- Shibata, M., Ohkubo, T., Takahashi, H. and Inoki, R. (1989). Modified formalin test: characteristic biphasic pain response. Pain 38(3): 347-352.
- Taylor, B. K., Peterson, M. A. and Basbaum, A. I. (1995). Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J Neurosci 15(11): 7575-7584.
- Zhang, J. Y., Gong, N., Huang, J. L., Guo, L. C. and Wang, Y. X. (2013). Gelsemine, a principal alkaloid from Gelsemium sempervirens Ait., exhibits potent and specific antinociception in chronic pain by acting at spinal alpha3 glycine receptors. Pain 154(11): 2452-2462.
- Zhu, B., Gong, N., Fan, H., Peng, C. S., Ding, X. J., Jiang, Y. and Wang, Y. X. (2014c). Lamiophlomis rotata, an orally available tibetan herbal painkiller, specifically reduces pain hypersensitivity states through the activation of spinal glucagon-like peptide-1 receptors. Anesthesiology 121(4): 835-851.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gong, N., Huang, Q., Chen, Y., Xu, M., Ma, S. and Wang, Y. (2014). Pain Assessment Using the Rat and Mouse Formalin Tests. Bio-protocol 4(21): e1288. DOI: 10.21769/BioProtoc.1288.
-
Gong, N., Xiao, Q., Zhu, B., Zhang, C. Y., Wang, Y. C., Fan, H., Ma, A. N. and Wang, Y. X. (2014b). Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J Neurosci 34(15): 5322-5334.
Category
Neuroscience > Sensory and motor systems
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









