- EN - English
- CN - 中文
Testing Bone Formation Induction by Calvarial Injection Assay in vivo
体内颅骨注射分析检测骨形成
发布: 2020年03月20日第10卷第6期 DOI: 10.21769/BioProtoc.3560 浏览次数: 5199
评审: Nicole HorwoodAntoine de MorreeAnonymous reviewer(s)

相关实验方案

采用 Davidson 固定液和黑色素漂白法优化小鼠眼组织切片的免疫组化染色
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
2025年11月20日 1573 阅读
Abstract
Bone formation occurs during embryogenesis, skeletal growth and during the process of skeletal renewal throughout life. In the process of bone formation, osteoblasts lay down a collagen-containing matrix, termed osteoid, which is gradually hardened by incorporation of mineral crystals. Although osteoblasts can be induced to differentiate and to deposit mineral in culture, this system does not always provide results that reflect the ability of agents to stimulate bone formation in vivo. This protocol describes a rapid and reliable method for testing local administration of agents on bone formation in vivo. In this method, mice are injected with the agent of question for 5 successive days. Fluorochrome labels are injected prior to, and after agents used for testing, and samples are collected and analysed by undecalcified bone histology and histomorphometry. This provides a robust method for assessing the ability of agents to stimulate bone formation, and if a short-term modification is used, can also be used for testing gene responses in bone to the same stimuli.
Keywords: Osteoblast (成骨细胞)Background
Bone formation is a fundamental process required for the development of the skeleton, for the progression of skeletal growth and to maintain bone structure throughout life as the skeleton is constantly remodeled. Bone formation is a process that occurs by three steps: (1) deposition of collagen-rich osteoid, (2) mineralization of that osteoid which occurs by deposition of bioapatite crystals around the collagen fibres, and (3) maturation of the mineralized bone substance (Blank and Sims, 2019). These three steps are mediated by two cell types derived from a common progenitor. Osteoblasts deposit osteoid and initiate mineralization, and as the osteoid is deposited, some osteoblasts become embedded within the bone matrix and differentiate into osteocytes (Dallas and Bonewald, 2010); these embedded cells also control the extent and nature of mineralization of bone (Vrahnas et al., 2019).
Identifying agents that can stimulate bone formation is critical for understanding the biology of the skeleton, and for developing new agents that can be used to promote bone formation in conditions of bone fragility such as osteoporosis, osteogenesis imperfecta, and osteomalacia, and to develop agents that can promote fracture healing or to stimulate bone formation in surgical interventions. Typically, the simplest system to test such agents is to use primary cultured osteoblasts (Orriss et al., 2014), or to differentiate stromal cell lines such as MC-3T3-E1 (Quarles et al., 1992) and Kusa 4b10 cells (Allan et al., 2003) in osteoblastogenic media. These systems are commonly used to study osteoblast differentiation by assessing effects of various agents on mRNA levels of osteoblast marker genes, alkaline phosphatase enzyme activity, and mineral deposition (Allan et al., 2003; McGregor et al., 2010; Walker et al., 2010). However, these systems do not always reflect the in vivo response. A clear example is the contrast between early in vivo works showing that leukemia inhibitory factor stimulates bone formation (Metcalf and Gearing, 1989; Cornish et al., 1993), and in vitro tests in which leukemia inhibitory factor could both stimulate and inhibit osteoblast differentiation (Malaval et al., 1995; Malaval and Aubin, 2001; Malaval et al., 2005; Falconi and Aubin, 2007).
In this protocol, we describe the use of a calvarial injection model which tests the ability of agents to stimulate bone formation in vivo. This method requires only small amounts of the stimulatory agent, and was first described by Cornish (Cornish et al., 1993) to resolve the controversy of whether leukemia inhibitor factor could promote bone formation. We have adapted the Cornish method to include calcein labelling to allow measurement of bone formation, and we have made use of it to test a range of cytokines including oncostatin M (Walker et al., 2010), cardiotrophin-1 (Walker et al., 2008), and most recently IL-6 acting through its soluble receptor (McGregor et al., 2019). We have also used this method to determine whether responses to cytokines are modified in mice with a cell-specific deletion of gp130, the common receptor used by these cytokines (Johnson et al., 2014). We provide here a full description of how to carry out the in vivo protocol, and how to embed and section tissues using undecalcified histology techniques; micro-computed tomography can also be used for assessment, but this does not allow measurement of bone formation rate using calcein labels. An abbreviated form of this method using single calvarial injections or two days of calvarial injections can also be used to assess gene responses elicited by cytokines in vivo by Western blot (Walker et al., unpublished) and effects on protein expression by immunohistochemistry (Walker et al., 2010).
Materials and Reagents
- Plastic flat-bottomed box
- Plastic zip-loc bag
- Hammer
- Thick rubber gloves
- Glass bottles for reagents–the methyl-methacrylate will dissolve plastic
- Microscope slides (Hurst scientific catalog number: 7107 )
- Coverslips (Hurst scientific catalog number: CG12450 )
- Glass Scintillation Vials (PerkinElmer catalog number: 6000097 )–methyl-methacrylate will dissolve plastic
- Grinding paper (smooth) CarbiMet S SiC P400 (ThermoFisher, catalog number: 16080320 )
- Grinding paper (rough) CarbiMet S, 60 P60 (ThermoFisher, catalog number: 16080060 )
- Insulin syringe (29 G BD Ultra-fineTM Clifford Hallam, catalog number: 1323684 )
- Microfuge tubes 1.5 ml (Pacific labs, Axygen catalog number: MCT175CI )
- 6-week old C57BL/6 mice (10 mice per group)
- Positive Control: Recombinant mouse Oncostatin M (OSM) (R&D Systems catalog number: 495-MO-025 ) with 2% heat-inactivated mouse serum (made in house)
- Your preferred agents/treatments for testing
- Phosphate buffered saline (Sigma-Aldrich, catalog number: D8537 )
- Isoflurane inhalant anaesthetic (FORTHANE®, AbbVie)
- Ethanol
- Calcein (Sigma-Aldrich, catalog number: C0875-5G )
- Sodium Bicarbonate (Sigma-Aldrich, catalog number: S6014 )
- Sodium hydroxide (NaOH)
- Acetone
- Paraformaldehyde (Sigma-Aldrich, catalog number: 158127-500 g )
- Methyl methacrylate (Sigma-Aldrich, catalog number: M55909-500 ml )
- Destabilised methylmethacrylate (dMMA)
- Dibutyl Phthalate (Merck, catalog number: 800919 )
- Granular calcium choride anhydrous (Sigma-Aldrich, catalog number: C-1016 )
- Chromium (III) potassium sulphate (Sigma-Aldrich, catalog number: 243361 )
- Gelatin from bovine skin (Sigma-Aldrich, catalog number: G9391 )
- Butanol (Merck, catalog number: 8222641000 )
- Toluene (Crown Scientific, catalog number: 2867322 )
- Depex mountant (Crown Scientific, catalog number: 360294H )
- Tris (Astral Scientific, catalog number: BIO3094T)
- Xylenol orange (Sigma-Aldrich, catalog number: X3500 )
- Xylenol Orange Stain (see Recipes)
Equipment
- 100 ml beaker
- 2 L glass separating funnel
- Fume hood
- Incubator
- Microwave oven
- Grinder/Polisher (Buehler Phoenix beta, or equivalent)
- Automated microtome, RM2265 Leica, equipped with tungsten carbide blade, or equivalent microtome
- Fluorescence microscope equipped with Osteomeasure image analysis system (or equivalent), (Osteometrics, https://www.osteometrics.com/)
Software
- Osteomeasure histomorphometry system (Osteometrics, https://www.osteometrics.com/), or equivalent
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- McGregor, N. E., Poulton, I. J., Walker, E. C. and Sims, N. A. (2020). Testing Bone Formation Induction by Calvarial Injection Assay in vivo. Bio-protocol 10(6): e3560. DOI: 10.21769/BioProtoc.3560.
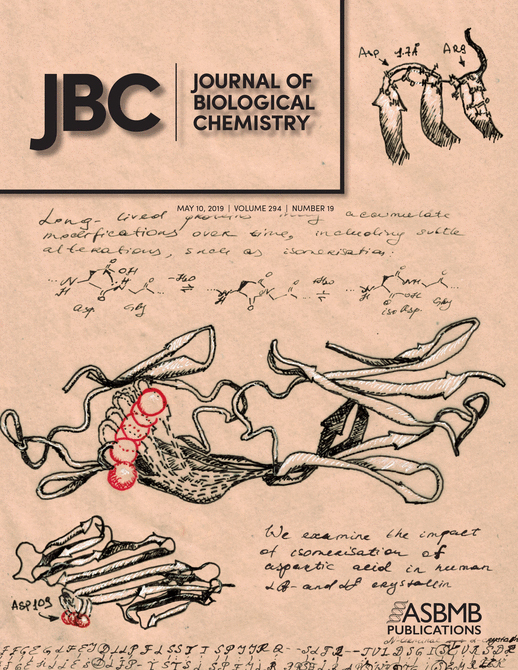
- McGregor, N. E., Murat, M., Elango, J., Poulton, I. J., Walker, E. C., Crimeen-Irwin, B., Ho, P. W. M., Gooi, J. H., Martin, T. J. and Sims, N. A. (2019). IL-6 exhibits both cis- and trans-signaling in osteocytes and osteoblasts, but only trans-signaling promotes bone formation and osteoclastogenesis. J Biol Chem 294(19): 7850-7863.
分类
发育生物学 > 细胞生长和命运决定 > 再生
细胞生物学 > 细胞染色 > 全细胞
细胞生物学 > 组织分析 > 组织形态学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link