- EN - English
- CN - 中文
Qualitative in vivo Bioluminescence Imaging
体内定性生物发光成像技术
发布: 2018年09月20日第8卷第18期 DOI: 10.21769/BioProtoc.3020 浏览次数: 10950
评审: Nicoletta CordaniLaura J. LambertAnonymous reviewer(s)
Abstract
Bioluminescence imaging (BLI) technology is an advanced method of carrying out molecular imaging on live laboratory animals in vivo. This powerful technique is widely-used in studying a variety of biological processes, and it has been an ideal tool in exploring tumor growth and metastatic spread in real-time. This technique ensures the optimal use of laboratory animal resources, particularly the ethical principle of reduction in animal use, given its non-invasive nature, ensuring that ongoing biological processes can be studied over time in the same animal, without the need to euthanize groups of mice at specific time points. In this protocol, the luciferase imaging technique was developed to study the effect of co-inoculating pericytes (contractile, αSMA+ mesenchymal stem cell-like cells, located abluminally in microvessels) on the growth and metastatic spread of ovarian cancers using an aggressive ovarian cancer cell line–OVCAR-5–as an example.
Keywords: Bioluminescence (生物发光)Background
The principle of bioluminescence imaging (BLI) is based on the light-emitting properties of a relatively simple biochemical process, i.e., luciferase-mediated oxidation of the molecular substrate luciferin to produce light. In cancer research, BLI is a popular tool (Contag et al., 2000) used to study the metastatic spread of luciferase-transduced cancer cells in live animals in vivo. Most animal tissues have little to no baseline bioluminescent properties, which ensures that there is a very high signal to noise ratio in BLI experiments. Nevertheless, it is always pragmatic to ensure that BLI experiments are conducted with non-substrate injected rodents as a negative control group. A few crucial factors are key to ensuring best practice in performing BLI experiments on rodents for the detection of luciferase-tagged cancer cells. Firstly, BLI is a powerful tool that allows easy detection of luciferase expressing-cells by imaging emitted bioluminescence under anesthesia (Figure 1). However, this technique is essentially qualitative and attempts to quantitate BLI signal can be misleading given that the strength of signal is dependent on several factors including duration of exposure, anesthetic technique, time elapsed after injecting luciferin, etc. Moreover, published evidence indicates that light emission (i.e., quantity of photons) is not directly related to luciferase activity (Rice et al., 2001). Secondly, the location or depth of the tissue of interest, particularly its distance from the skin’s surface, and the size of the metastatic cell mass are important considerations in planning a BLI experiment (Weissleder, 2001). This is because photon loss occurs as the signal travels through the tissue mass–consequently, luciferase-tagged cells/tissues that are closer to the skin’s surface tend to appear brighter as do larger metastases. Micrometastases can be detected but may require sacrificing the animal and imaging the organs directly after skin removal in place of intact animals. Notwithstanding these and other challenges, BLI is an efficient and powerful, non-invasive technique for studying biological processes in vivo.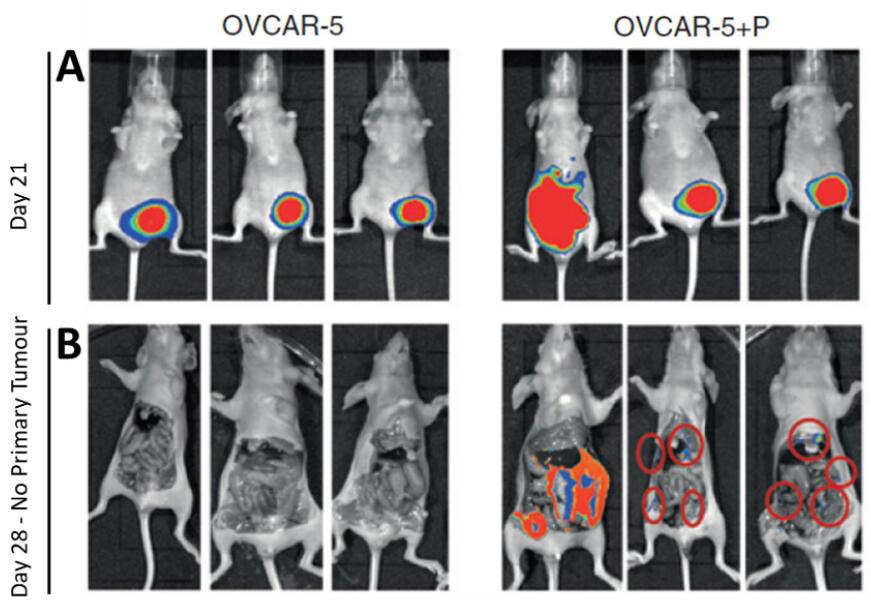
Figure 1. Imaging primary subcutaneous OVCAR-5 tumors and metastases. A. GFP-luciferase labeled OVCAR-5 cells, xenografted alone (OVCAR-5) or co-injected with pericytes (OVCAR-5+P) generated tumors that were imaged at regular intervals. The increased BLI signal observed in images of pericyte co-injected xenografts show that pericytes promote OVCAR-5 tumor growth rate and induce metastases compared to the control group. B. At day 28 the BLI signal from the primary tumor is saturated, and the mice were sacrificed and the primary tumors (along with the abdominal skin excised to permit clearer imaging of the metastatic nodules–metastatic nodules marked with red rings).
Materials and Reagents
- Materials
- Pipette tips (Interpath Services, catalog numbers: 39770 , 39730 )
- Hamilton® syringe (Hamilton, catalog number: 80366 )
- Tissue culture plasticware (6-well plates BD; 2, 5 and 10 ml pipettes SARSTEDT)
- Steritop-GP polyethersulfone with low binding PES membrane (0.22 μm pore size) (Merck, Millipore, catalog number: SCGPS05RE )
- Pasteur pipette (Biologix, catalog number: 30-0138A1 )
- Biological materials
- Lentiviral vector pFUGW-Pol2-ffLuc2-eGFP (Addgene, catalog number: 71394 )
- OVCAR-5 cell line was obtained from NCI, and authenticated using short tandem repeat markers to confirm cell identity against the Genome Project Database (Wellcome Trust Sanger Institute)
- HIV-1 packaging vector pCMV-deltaR8.2 (Addgene, catalog number: 8455 ), a kind gift from Dr. Cameron Johnstone, Anderson Lab, Peter MacCallum Cancer Centre, Melbourne
- Packaging cell line HEK293T (ATCC, catalog number: CRL-3216 ), a kind gift from Dr. Cameron Johnstone, Anderson Lab, Peter MacCallum Cancer Centre, Melbourne
- Mice: 6-8 weeks old female athymic nude Balb/c mice were obtained from Walter Eliza Hall Institute, housed in a pathogen-free 12 h light–dark environment, fed ad libitum were used for tumorigenicity assays. This age range of mice is optimal for good tumor take rates which decline if older mice (e.g., 10 weeks old) are used.
- Reagents
- Bovine serum albumin (Sigma-Aldrich, catalog number: A9418-500G )
- DAPI:4’, 6’-Diamidino-2-Phenylindole Dihydrochloride (Sigma-Aldrich, catalog number: D9542-5MG )
- DMEM (Thermo Fisher Scientific, GibcoTM, catalog number: 11965092 )
- Diflucan (Fluconozole) (Sigma-Aldrich, catalog number: F8929 )
- D-Luciferin: Sodium salt 4,5-Dihydro-2-(6-hydroxy-2-benzothiazolyl)-4-thiazolecarboxylic acid sodium salt (Gold Biotechnology, catalog number: LUCNA-1G )
- FuGENE 6 (Roche Diagnostics, Mannheim, Germany)
- Endothelial basal media (EBMTM-2) (Lonza, catalog number: CC-3156 )
- Endothelial growth media (EGMTM-2) SinglequotsTM (Lonza, catalog number: CC-4147 )
- Fetal Calf Serum (FCS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10099141 )
- Forthane/Isoflurane (Sigma-Aldrich, catalog number: 792632 )
- HEPES pH 7.4 (Sigma-Aldrich, catalog number: H0887-100ML )
- MatrigelTM (standard) (BD, catalog number: 356234 )
- PBS without Ca2+ and Mg2+ (GE Healthcare, Hyclone, catalog number: SH30256.FS )
- Penicillin-Streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Polybrene (Sigma-Aldrich, catalog number: 107689 )
- Potassium chloride (Sigma-Aldrich, catalog number: P5405 )
- Potassium dihydrogen phosphate (Sigma-Aldrich, catalog number: P5655 )
- RMPI-1640 (Thermo Fisher Scientific, InvitrogenTM, catalog number: 11875 )
- Sodium bicarbonate (Sigma-Aldrich, catalog number: S5761 )
- Sodium chloride (Astral Scientific, catalog number: AMX190 )
- Sodium hydrogen phosphate (Sigma-Aldrich, catalog number: S5136 )
- Trypsin 0.05% (Thermo Fisher Scientific, GibcoTM, catalog number: 25300120 )
- Trypan blue (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
- Phosphate buffered Saline (PBS) (see Recipes)
- RPMI-1640 media (see Recipes)
Equipment
- Pipettes (Corning, catalog numbers: 4487 , 4488 , 4489 )
- Hemocytometer (ProSciTech, catalog number: SVZ2NI0U )
- Cell culture incubator (NuAire, model: NU-5510/E )
- Centrifuge (Beckman Coulter, model: Allegra X-12 )
- Electronic calipers (Fisher Scientific, catalog number: 14-648-17 )
- SW28 Rotor (Beckman Coulter, catalog number: 342207 )
- Ultracentrifuge (Beckman Coulter, model: OptimaTM XE-100 )
- Fluorescent Microscope (Nikon Instruments, model: Nikon A1+ Confocal Microscope )
- Xenogen Realtime Imaging System (IVIS Lumina II)
- Becton Dickinson Biosciences FACS DivaTM cell sorter
Software
- Prism 6 (GraphPad Inc.)
- Photoshop CS 6.0 (Adobe Inc.)
- Metamorph (Molecular devices)
- ImageJ (NIH software)
Procedure
文章信息
版权信息
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Sinha, D., Pieterse, Z. and Kaur, P. (2018). Qualitative in vivo Bioluminescence Imaging. Bio-protocol 8(18): e3020. DOI: 10.21769/BioProtoc.3020.
分类
癌症生物学 > 侵袭和转移 > 动物模型 > 细胞侵袭
干细胞 > 成体干细胞 > 上皮干细胞
细胞生物学 > 细胞工程 > 慢病毒递送
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













