Advanced Search
Serological analysis
Last updated date: Mar 25, 2021 Views: 964 Forks: 0
SARS-CoV-2 specific IgA, IgM and IgG antibodies were measured in 214 serum samples from 132 patients with The Maverick ™ SARS-CoV-2 Multi-Antigen Serology Panel (Genalyte Inc. USA) according to the manufacturer’s instructions (please find enclosed reagents datasheet). The Maverick ™ SARS-CoV-2 Multi-Antigen Serology Panel (Genalyte Inc) is designed to detect antibodies to five SARS-CoV-2 antigens: nucleocapsid, Spike S1 RBD, Spike S1S2, Spike S2 and Spike S1, with in a multiplex format based on photonic ring resonance technology (18, 19). This automated system detects and measures with good reproducibility (Figure S3) changes in resonance when antibodies bind to their respective antigens on the chip. Combined IgG and IgM antibodies showed 91% sensitivity and 98% specificity. Briefly, 10 µl of each serum sample was added to a sample well plate array containing required diluents and buffers, and the plate and chip were loaded in the instrument for chip equilibration with the diluent buffer to measure baseline resonance. The serum sample is then charged over the chip to bind specific antibodies to antigens present on the chip. The chip is then washed to remove low affinity binders, and specific antibodies are detected with anti-IgG, -IgA or -IgM secondary antibodies. 43 sera collected before December 2019 were analyzed to calculate cut-off values. Positivity was defined as results above the 99th percentile.
Maverick™ SARS-CoV-2 Multi-Antigen Serology Panel v2 01030ART-01
For prescription use only.
For in vitro diagnostic use only.
For Emergency Use Authorization only.
Intended Use
The Maverick SARS-CoV-2 Multi-Antigen Serology Panel v2 is a photonic ring immunoassay (PRI) intended for qualitative detection of total antibodies (including IgG and IgM) to SARS-CoV-2 in human dipotassium EDTA venous whole blood, dipotassium EDTA plasma, and serum using the MaverickTM Diagnostic System. The Maverick SARS-CoV-2 Multi-Antigen Serology Panel v2 is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The Maverick SARS-CoV-2 Multi- Antigen Serology Panel v2 should not be used to diagnose acute SARS-CoV-2 infection. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C 263a, that meet requirements to perform moderate or high complexity tests.
Results are for the detection of total SARS CoV-2 antibodies. Antibodies to SARS-CoV-2 are generally detectable in blood several days after initial infection, although the duration of time antibodies are present post-infection is not well characterized. Individuals may have detectable virus present for several weeks following seroconversion.
Laboratories within the United States and its territories are required to report all results to the appropriate public health authorities.
The sensitivity of the Maverick SARS-CoV-2 Multi-Antigen Serology Panel v2 early after infection in unknown. Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
False positive results for Maverick SARS-CoV-2 Multi-Antigen Serology Panel v2 may occur due to cross-reactivity from pre-existing antibodies or other possible causes. Due to the risk of false positive results, confirmation of positive results should be considered using a second, different total antibody assay.
The Maverick SARS-CoV-2 Multi-Antigen Serology Panel v2 is only for use under the Food and Drug Administration's Emergency Use Authorization.
Summary and Explanation of the Test
The SARS-CoV-2 Multi-Antigen Serology Panel v2 is a kit that allows a patient sample to be tested for the presence of total antibodies to SARS-CoV-2.
Principles of the Procedure
Genalyte has developed multiplex detection technology based on silicon photonics that uses ring resonance to measure binding of macromolecules to sensors on a miniature silicon chip. The Maverick Diagnostic System detects changes in resonance wavelength as macromolecules, such as antibodies, bind to their respective antigens that are bound to the chip. The antigens include 5 SARS-CoV-2 proteins, 4 proteins (one each) to the four human benign coronaviruses, two influenza hemagglutinins, MERS, and SARS-CoV-1 coronavirus proteins (see Table1). The patient sample is added to a specific well of a reagent plate which contains the required diluents and buffers. The plate and the chip carrier are loaded into the Maverick instrument. The instrument automates the assay and takes the diluted sample from the reagent plate and flows the sample over the silicon chip allowing any SARS-CoV-2, common coronavirus, influenza hemagglutinin, SARS-CoV-1, and MERS coronavirus antibodies present in the patient sample to bind to the immobilized antigens. Unbound sample is washed away, and then, in succession goat anti-human IgG and goat anti-human IgM are flowed over the chip to detect the specific class of antibodies bound to any antigens on the chip. The signal is the difference between the successive baseline measurements in GRU (Genalyte Response Units).
A multi-antigen analysis algorithm analyzes individual qualitative results in order to generate an overall positive or negative result for antibodies to SARS-CoV-2. Results for the antibodies to antigens other than SARS-CoV-2 (common coronavirus, influenza hemagglutinin, SARS-CoV-1, and MERS) will not be reported.
Chip Carrier and Plate:

Table 1. Proteins included on SARS-CoV-2 Multi-Antigen Serology Panel v2 (Individual Data not Reported)
| Virus | Antigen |
| SARS-CoV-2 | Nucleocapsid |
| SARS-CoV-2 | Spike Protein - full length |
| SARS-CoV-2 | Spike Protein - S1 subunit |
| SARS-CoV-2 | Spike Protein - S2 subunit |
| SARS-CoV-2 | Spike Protein - S1 RBD |
| SARS-CoV-229E | Spike Protein |
| SARS-CoV-NL63 | Nucleoprotein |
| SARS-CoV-OC43 | Spike Protein |
| SARS-CoV-HKU1 | Spike Protein |
| Influenza A | Hemagglutinin H1 |
| Influenza A | Hemagglutinin H3 |
| MERS | Spike Protein S1 subunit |
| SARS-CoV-1 | Nucleocapsid |
Reagents and Materials
Silicon Chip (PN 01030): spotted with viral antigens, and quality control proteins housed in a carrier in foil pouch with desiccant.
Additional materials packaged separately:
Pregen Reagent Plate (PN 00993): sealed, prefilled in a foil sealed plate containing the following reagents:
- Running Buffer (wells A1, A2), clear liquid containing PBS, Tween®-20, proprietary proteins, and preservative.
- Regeneration Buffer (wells B-F, 1&2), clear liquid containing glycine and SDS Solution
- TE Buffer (wells G1, G2), clear liquid Tris-EDTA buffer
Reagent Plate (PN 01031): sealed, prefilled in a foil sealed plate containing the following reagents:
- Running Buffer (wells A-D, 1&2), clear liquid containing PBS, Tween®-20, proprietary proteins, and preservative.
- SARS-CoV-2 Multi-Antigen Serology Panel Detection Buffer-1 (wells E1, E2), clear liquid containing goat anti-human IgG, PBS, Tween®-20, proprietary proteins, and preservative.
- SARS-CoV-2 Multi-Antigen Serology Panel Detection Buffer-2 (wells F1, F2), clear liquid containing goat anti-human IgM, PBS, Tween®-20, proprietary proteins, and preservative.
- Regeneration Buffer (wells G1, G2), clear liquid containing glycine and SDS Solution.
- TE Buffer (wells H1, H2), clear liquid Tris-EDTA buffer.
Calibrator (PN 00990): Store calibrator at -20 °C or lower. Once thawed, store the vial at 2-8 °C for no longer than 7 days.
External Quality Controls: Store all controls at -20 °C or lower. Once thawed, store the vial at 2-8 °C for no longer than 7 days.
It is recommended to use PC1 and NC as daily controls for the instrument and assay.
- SARS-CoV-2 Multi-Antigen Serology Panel Positive Control-1 (PC-1, Genalyte part number: 00983)
- SARS-CoV-2 Multi-Antigen Serology Panel Negative Control (NC, Genalyte part number: 00984)
Additional Materials Required - Not Provided Maverick Diagnostic System with operating computer Pipettes able to deliver 5µL - 200µL
Conditioning sample utilized during chip initialization procedure
Warnings
- This test has not been FDA cleared or approved; this test has been authorized by FDA under an EUA for use by laboratories certified under CLIA, that meet requirements to perform moderate or high complexity tests.
- This test has been authorized only for the presence of total antibodies against SARS-CoV-2, not for any other viruses or pathogens.
- This test is only authorized for the durationof the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb3(b)(1), unless the authorization is terminated or revoked sooner.
- Sodium azide is used as a preservative. Sodium azide is a poisonand may be toxic if ingested or absorbed through the skin or eyes. Sodium azide may react with lead or copper plumbing to form potentially explosive metal azides. Flush sinks, if used for reagent disposal, with large volumes of water to prevent azide build-up.
- Use appropriate personal protective equipment while working with the reagents provided.
- Spilled reagents should be cleaned up immediately. Observe all federal, state, and local environmental regulations when disposing of waste.
- Used chips and stripwells should be disposed of as a biohazard. Observe all federal, state, and local environmental regulations when disposing of waste.
Precautions
- Microbially contaminated, heat-treated, or specimens containing visible particulates should not be used.
- Follow universal precautions when handling reagent plates, calibrators, external controls, and specimens.
- Do not use a pregen or reagent plate if the plate is leaking or the foil has been pierced prior to loading calibrator, control, or patient sample, if applicable.
- Do not use expired kits, plates, or reagents.
Limitations
- This assay is only for use with the Maverick Diagnostic System and associated CloudLab software.
- This assay has not been evaluated with fingerstick specimens. This test is not authorized for use with fingerstick whole blood.
- The test is only to be used in CLIA certified laboratories and not in point-of-care or at-home testing settings.
- Use of SARS-CoV-2 Multi-Antigen Serology Panel v2 is limited to laboratory personnel who have been trained. Not for home use.
- Performance has only been established with the specimen types listed in the Intended Use. Other specimen types have not been evaluated and should not be used with this assay.
- It is not known at this time if the presence of antibodies to SARS-CoV-2 confers immunity to re-infection.
- SARS-CoV-2 antibodies may be below detectable levels in serum samples collected from patients who have been exhibiting symptoms for less than 8 days or in venous whole blood or plasma samples collected from patients who have been exhibiting symptoms for less than 15 days.
- Testing with a molecular diagnostic should be performed to evaluate for acute SARS-CoV-2 infection in symptomatic individuals.
- Results from antibody testing should not be used to diagnose or exclude acute COVID-19 infection or to inform infection status.
- A negative result for an individual subject indicates absence of detectable anti-SARS-CoV-2 antibodies. Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. The sensitivity of this assay early after infection is unknown.
- False positive results may occur due to cross-reactivity from pre-existing antibodies or other possible causes.
- A positive result may not indicate previous SARS-CoV-2 infection. Consider other information including clinical history and local disease prevalence, in assessing the need for a second but different serology test to confirm an immune response. Positive results must be confirmed with another available method and interpreted in conjunction with the patient's clinical information.
- A negative result can occur if the quantity of the anti-SARS-CoV-2 antibodies present in the specimen is below the detection limits of the assay, or the antibodies that are detected are not present during the stage of disease in which a sample is collected.
Conditions of Authorization for the Laboratory
The Maverick SARS-CoV-2 Multi-Antigen Serology Panel v2 Letter of Authorization, along with the authorized Fact Sheet for Healthcare Providers, the authorized Fact Sheet for Patients, and authorized labelling are available on the FDA website: https://www.fda.gov/medical- devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical- devices/vitro-diagnostics-euas or at www.genalyte.com.
Authorized laboratories using the Maverick SARS-CoV-2 Multi-Antigen Serology Panel v2 (“your product” in the conditions below), must adhere to the Conditions of Authorization indicated in the Letter of Authorization as listed below:
- Authorized laboratories* using your product will include with result reports of your product, all authorized Fact Sheets. Under exigent circumstances, other appropriate methods for disseminating these Fact Sheets may be used, which may include mass media.
- Authorized laboratories using your product will use your product as outlined in the Instructions for Use. Deviations from the authorized procedures, including the authorized instruments, authorized clinical specimen types, authorized control materials, authorized other ancillary reagents and authorized materials required to use your product are not permitted.
- Authorized laboratories that receive your product will notify the relevant public health authorities of their intent to run your product prior to initiatingtesting.
- Authorized laboratories using your product will have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
- Authorized laboratories will collect information on the performance of your product and report to DMD/OHT7-OIR/OPEQ/ CDRH (via email: CDRH-EUA- Reporting@fda.hhs.gov) and Genalyte, Inc. (feedback@genalyte.com) any suspected occurrence of false reactive or false non-reactive results and significant deviations from the established performance characteristics of your product of which they become aware.
- All laboratory personnel using your product must be appropriately trained in automated immunoassay techniques and use appropriate laboratory and personal protective equipment when handling this kit and use your product in accordance with the authorized labeling. All laboratory personnel using the assay must also be trained in and be familiar with the interpretation of results of the product.
- Genalyte, Inc., authorized distributors, and authorized laboratories using your product will ensure that any records associated with this EUA are maintained until otherwise notified by FDA. Such records will be made available to FDA for inspection upon request.
*The letter of authorization refers to, “Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform moderate or high complexity tests” as “authorized laboratories.”
Storage Conditions
- Store SARS-CoV-2 Multi-Antigen Serology Panel v2 kit components and reagents at 2-8 °C. Do not freeze. Reagents are stable until the expiration date when stored and handled as directed.
- SARS-CoV-2 Multi-Antigen Serology Panel v2 assay reagent plate (PN 01030) must be stored at 2-8℃ until use. Discard after use.
- Store SARS-CoV-2 Multi-Antigen Serology Panel v2 calibrator and external quality controls at least -20°C until open. Once open, the calibrator and external quality controls are stable for 7 days at 2-8 °C.
Specimen Collection
This procedure may be performed using EDTA venous whole blood, plasma or serum specimens. Microbially contaminated, heat-treated, or specimens containing visible particulates should not be used. Lipemic or icteric specimens should not be used. Storage conditions for samples are as follows:
- Specimens should be tested as soon as possible after collection.
- Store EDTA anticoagulant venous whole blood at 2-8 °C if not tested immediately. Do not freeze whole blood.
- Store serum and EDTA plasma at -20 °C if not tested immediately. Avoid multiple freeze/thaw cycles.
Using the Maverick Diagnostic System
Refer to the Maverick Diagnostic System operator’s manual for detailed operating instructions of the Maverick system and software. For additional information and for troubleshooting problems with this assay, contact Genalyte, Inc. customer service at feedback@genalyte.com.
Before you start
Allow all samples and reagents to come to room temperature (20-26°C) prior to use.
Maverick Diagnostic System Set-Up
- Power on the Maverick instrument, computer, and monitor.
- Log into the system by scanning in user credentials.
- Scan the 2D barcode located on the SARS-CoV-2 Multi-Antigen Serology Panel v2 kit label. The following fields will populate:
- Kit Part No.
- Kit Lot No.
- Kit Serial No.
- Kit Exp.
Conditioning Protocol
- Carefully remove the chip carrier from the foil packaging. Note: Avoid contact with the carrier sippers.
- Insert the chip carrier into the Maverick Diagnostic System.
- Insert the chip carrier, sippers pointing down and away from the instrument.

- Use the metal guide ledges and slide the carrier in until a hard stop is obtained.

3. Prepare a room temperature reagent plate (PN 01031).
4. “Flick” the plate to ensure all reagents are on the bottom of the wells.
5. A Conditioner sample (any serum sample) is diluted in the running buffer that is preloaded into the reagent plate. Pierce foil and add 10µL of the initialization sample to wells A1 and A2 of the reagent plate. Mix well by pipetting up and down 10 times using a pipette with a set volume of 50 µL.
6. Scan the barcode on the reagent plate into the Maverick. Click “OK”.
7. Scan the conditioner barcode on the vial. Click “Use same ID in both channels”.
8. Load the reagent plate into the instrument by orienting the plate with the notches toward the instrument, and blue line toward the operator. Slide the reagent plate into the plate carriage, notched end first. Note: To avoid splashing or creating bubbles, hold the plate with both hands and ease into the instrument until the plate engages.
9. Once the reagent plate is loaded into the instrument, close the instrument door.
10. Click “Start Test”, then click “Yes” to start the run. Note: Once the Maverick is running, do not open the door until the assay is complete.
11. A timer will be displayed on screen counting down until the protocol is complete. The protocol will run for approximately 20 minutes.
12. Once the software indicates that the run is complete, remove the consumed reagent plate and discard. Leave the chip in the carrier in theinstrument.
13. Proceed to Pregen by clicking “New Test”.
Pregen Protocol
- Prepare a room temperature Pregen reagent plate (PN00993).
- “Flick” the plate to ensure all reagents are on the bottom of the wells.
- Load the Pregen reagent plate into the instrument by orienting the plate with the notches toward the instrument, and holes toward the operator. Slide the reagent plate into the plate carriage, notched end first. Note: To avoid splashing or creating bubbles, hold the plate with both hands and ease into the instrument until the plate engages.
- Once the reagent plate is loaded into the instrument, close the instrument door.
- Scan the QR code on the foil pouch. This will display a prompt asking if you want to continue with the test as a maintenance protocol is selected. Click “Yes”.
- Start the run by selecting “Yes.” Note: Once the Maverick is running, do not open the door until the assay is complete.
- A timer will be displayed on screen counting down until the Pregen protocol is complete. The Pregen protocol will run for approximately 10 minutes.
- Once the software indicates that the run is complete, remove the consumed pregen plate and discard. Leave the chip in the carrier in theinstrument.
- Proceed to calibration by clicking “New Test”.
Calibration Protocol
- Prepare a room temperature reagent plate (PN 01031).
- “Flick” the plate to ensure all reagents are on the bottom of the wells.
- Calibrators are diluted in the running buffer that is preloaded into the reagent plate. Pierce foil and add 10µL of the calibrator to wells A1 and A2 of the reagent plate. Mix well by pipetting up and down 10 times using a pipette with a set volume of 50 µL.
- Scan the barcode on the reagent plate into the Maverick. Click“OK”.
- Scan the calibrator barcode on the vial. Click “Use same ID in both channels”.
- Load the reagent plate into the instrument by orienting the plate with the notches toward the instrument, and blue line toward the operator. Slide the reagent plate into the plate carriage, notched end first. Note: To avoid splashing or creating bubbles, hold the plate with both hands and ease into the instrument until the plate engages.
- Once the reagent plate is loaded into the instrument, close the instrument door.
- Click “Start Test”, then click “Yes” to start the run. Note: Once the Maverick is running, do not open the door until the assay is complete.
- Once calibration is complete, the CLS monitor reviews the calibration data to ensure that the values for IgG and IgM reactivity to SARS-CoV-2 proteins are within acceptable limits. The acceptable ranges for SARS-CoV-2 reactivity from the calibrator are listed in Table 2. According to common statistical practices, the calibrator must have ≥7 of the 10 measurements within range to pass (IgG to 5 proteins and IgM to 5 proteins).
Table 2. Acceptable Ranges for SARS-CoV-2 Reactivity from the Calibrator
| CoV-2 S1 RBD |
CoV-2 S1 |
CoV-2 S2 | CoV-2 S1S2 |
CoV-2 NC | CoV-2 S1 RBD |
CoV-2 S1 |
CoV-2 S2 | CoV-2 S1S2 | CoV-2 NC | ||
| IgG Low | 84 | 41 | 93 | 45 | 151 | IgM Low | 142 | 39 | 6 | 9 | 13 |
| IgG High | 209 | 92 | 162 | 101 | 321 | IgM High | 249 | 92 | 28 | 26 | 36 |
10. If calibration does not pass, the CLS monitoring the assay and instrument performance can decide to rerun calibration as they deem appropriate. Based upon available data, the CLS monitor will decide if the calibration run(s) meet acceptability requirements and pass, or if the calibration run(s) fail.
11. Once calibration has passed, proceed to QC protocol.
12. If calibration has failed, remove and discard the chip. Obtain a new reagent plate and chip, scan the kit barcode, and start the sample initialization protocol.
External Control Protocol
- Prepare a room temperature reagent plate (PN 01031).
- “Flick” the plate to ensure all reagents are on the bottom of the wells.
- Scan or manually enter the control ID into the Maverick - Channel 1 and 2.
- External control samples are diluted in the running buffer that is preloaded in the reagent plate. Pierce foil and add 10µL of external control (Positive Control-1 or Negative Control) to wells A1 and A2 of the reagent plate. Mix well by pipetting up and down 10 times using a pipette with a set volume of 50 µL.
- Scan the barcode on the reagent plate into the Maverick.
- Load the reagent plate into the instrument by orienting the plate with the notches toward the instrument, and blue line toward the operator. Slide the reagent plate into the plate carriage, notched end first. Note: To avoid splashing or creating bubbles, hold the plate with both hands and ease into the instrument until the plate engages.
- Once the reagent plate is loaded into the instrument, close the instrument door.
- Click start. Note: Once the Maverick is running, do not open the door until the assay is complete.
- Refer to lot specific CoA for expected results.
- Repeat steps 1-8 for the second external control (a positive and negative control must be run in each channel).
- Once QC has passed the chip is now ready for use with reagent plates and approved specimen types.
- If QC does not pass, the CLS monitoring assay and instrument performance can decide to rerun either the PC-1 or NC as they deem appropriate (similar to any PC-1 or NC on an instrument with another assay). Based upon available data, the CLS monitor will decide if the QC run(s) meet acceptability requirements and pass, or if the QC run(s) fail.
- If QC has failed, remove and discard the chip. Obtain a new reagent plate and chip, scan the kit barcode, and start the sample initialization protocol.
Patient Sample Preparation
- Prepare a room temperature reagent plate (PN 01031).
- “Flick” the plate to ensure all reagents are on the bottom of the wells.
- Scan or manually enter the specimen ID into the Maverick – Channel 1 field.
- Patient sample for analysis is diluted in the running buffer that is preloaded in the reagent plate. Pierce foil and add 20 µL of whole blood (EDTA anticoagulant) or 10 µL of plasma or serum to well A1 of the reagent plate. Mix well by pipetting up and down 10 times using a pipette with a set volume of 50 µL.
- Scan or manually enter the specimen ID into the Maverick – Channel 2 field.
- Add 20 µL of whole blood with EDTA anticoagulant or 10 µL of plasma or serum to well A2 of the reagent plate. Mix well by pipetting up and down 10 times using a pipette with a set volume of 50 µL.
- Scan the barcode on the reagent plate into the Maverick.
- Load the reagent plate into the instrument by orienting the plate with the notches toward the instrument, and blue line toward the operator. Slide the reagent plate into the plate carriage, notched end first. Note: To avoid splashing or creating bubbles, hold the plate with both hands and ease into the instrument until the plate engages
- Once the reagent plate is loaded into the instrument, close the instrument door.
- Click Start. Note: Once the Maverick is running, do not open the door until assay is complete.
Run Completion
- Once the assay has been completed, a status message will show “Test Complete”.
- Open the door, remove and discard the reagent plate as a biohazard in accordance with local, state, and federal regulations.
- The chip in the carrier may be reused for multiple assay runs (limit of 25 per channel).
- When applicable, dispose of the chip in the carrier as a biohazard in accordance with local, state, and federal regulations.
Interpretation of Results
Description of Multi-Antigen Analysis Algorithm to Call Positive vs Negative SARS- CoV-2 Antibody Response
The MaverickTM SARS-CoV-2 Multi-Antigen Serology Panel v2 utilizes a multi-analyte analysis algorithm (MAAA) to make the determination on patient samples of positive or negative or indeterminate for antibodies to the SARS-CoV-2 virus. The algorithm employed is a well-known ensemble method called Random Forests Classification. Random Forests contain a number of decision trees constructed from randomly chosen features that each make predictions on the data set, the aggregation of which gives the final result. These models are capable of fitting complex datasets and are resistant to overfitting.
Our implementation of the method uses 3000 such decision trees sampled randomly from training data and validated against test data. The model was also cross validated using fivefold cross validation. Three models were trained, and the combined IgG and IgM model proved to be the most robust, and is the model carried forward to call patient samples positive, negative, or indeterminate for antibodies to SARS-CoV-2.
| Probability of positive score (not reported) | Result | Test result interpretation |
| 0.55 - 1.00 | Positive | Anti-SARS-CoV-2 antibodies are detected. |
| 0.451 - 0.549 | Indeterminate | |
| 0.0 - 0.45 | Negative | Anti-SARS-CoV-2 antibodies are not detected. |
Performance Characteristics
Training of Algorithm
755 presumptively normal samples collected prior to November 2019 and 243 samples that were confirmed PCR positive for SARS-CoV-2 from 243 subjects were used to train the MAAA. All negative samples were collected from rheumatology or primary care clinics in routine clinical care, under IRB. All positive samples were collected retrospectively from patients presenting in ambulatory clinics, with suspected COVID-19, and who underwent NP/OP swab confirmation of SARS-CoV-2.
Known SARS-CoV-2 positive samples
338 serum and plasma samples that were not used in MAAA training collected from 275 patients confirmed PCR positive for SARS-CoV-2 were tested with the SARS-CoV-2 Multi-Antigen Serology Panel v2. Samples were collected prospectively or retrospectively, and all patients were confirmed to be SARS-CoV-2 positive by PCR. The following table presents positive percent agreement by time from a positive PCR test. No more than one sample was collected from each patient in each time period.
Days from symptom onset | Number Tested | Positive | PPA | 95% CI |
| 0 - 7 | 69 | 46 | 66.67% | 54.93% – 76.65% |
| 8 - 14 | 88 | 80 | 90.91% | 83.07% - 95.32% |
| ≥15 | 181 | 174 | 96.13% | 92.23% - 98.11% |
| Total | 338 |
Presumptively SARS-CoV-2 negative samples
814 presumptively normal samples collected prior to November 2019 were utilized to validate the MAAA. These samples were independent from the training sample set, but collected from a similar patient cohort. In addition, 48 samples collected from patients confirmed to be SARS-CoV-2 negative by PCR were evaluated for a total of 862 negative samples tested.
| Number Tested | Negative | NPA | 95% CI |
| 862 | 842 | 97.68% | 96.44% - 98.49% |
Matrix Comparison
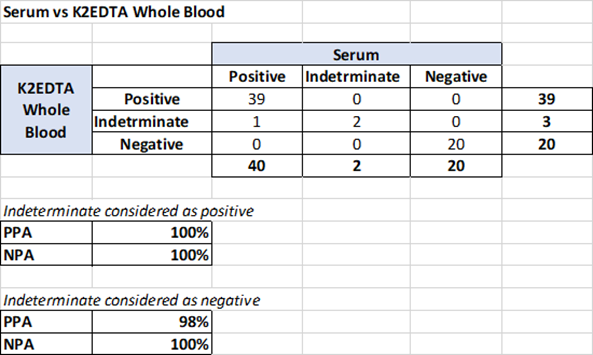
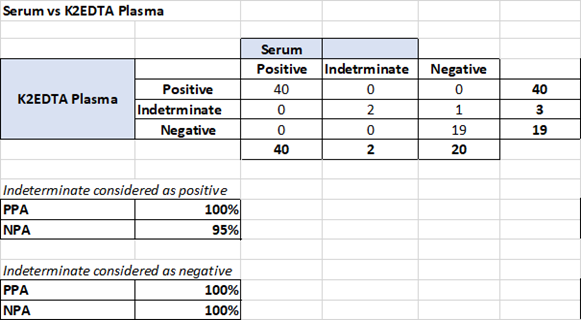
A total of 31 K2EDTA anticoagulated whole blood, plasma, and serum pairs, collected from patients at the same time tested in duplicates were compared: Data was generated by the machine learning algorithm as to overall positivity (combined IgG and IgM in model). Samples with low and high probabilities of positive results were included. The study supports equivalency of serum, K2EDTA whole blood, and K2EDTA plasma as matrices for samples tested with the MaverickSARS-CoV-2 Multi-Antigen Serology Panel v2.
Potential Cross Reactive Subsets
The MaverickTM SARS-CoV-2 Multi-Antigen Serology Panel v2 was evaluated for cross-reactivity in patients with autoimmune disease and in patients with infections with non-SARS-CoV-2 viruses. No cross reactivity was observed with the following:
| Condition | N of samples | Number Positive | Number Negative |
| Systemic Lupus Erythematosus | 5 | 0 | 5 |
| Rheumatoid Arthritis | 5 | 0 | 5 |
| Mixed Connective Tissue Disease | 5 | 0 | 5 |
| Scleroderma | 5 | 0 | 5 |
| Osteoporosis | 5 | 0 | 5 |
| Respiratory Syncytial Virus | 5 | 0 | 5 |
| Cytomegalovirus | 4 | 0 | 4 |
| Epstein Barr Virus | 3 | 0 | 3 |
| Hepatitis B Virus | 3 | 0 | 3 |
| Hepatitis C Virus | 4 | 0 | 4 |
- Miyara, M and Sterlin, D(2021). Serological analysis. Bio-protocol Preprint. bio-protocol.org/prep961.
- Sterlin, D., Mathian, A., Miyara, M., Mohr, A., Anna, F., Claër, L., Quentric, P., Fadlallah, J., Devilliers, H., Ghillani, P., Gunn, C., Hockett, R., Mudumba, S., Guihot, A., Luyt, C., Mayaux, J., Beurton, A., Fourati, S., Bruel, T., Schwartz, O., Lacorte, J., Yssel, H., Parizot, C., Dorgham, K., Charneau, P., Amoura, Z. and Gorochov, G.(2021). IgA dominates the early neutralizing antibody response to SARS-CoV-2. Science Translational Medicine 13(577). DOI: 10.1126/scitranslmed.abd2223
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
