Advanced Search
Protein expression, purification, and site-specific labeling
Last updated date: Feb 16, 2021 Views: 1025 Forks: 0
Reagents
- 5 M NaCl
- 1 M Tris-HCl, pH 8.0
- 2 M MgCl2
- Dithiothreitol (DTT)
- 0.075 % (w/v) digitonin. Solubilised stirring 12 hours at 4 ⁰C under argon gas, and filtered with a 0.22 µm filter.
- 50 % Glycerol
- 10 % (w/v) Lauryl maltoseneopentyl glycol (LMNG),1 % (w/v) cholesteryl hemisuccinate (CHS). Solubilised nutating 12 hours at 4 ⁰C under argon gas.
- NHS-activated Sepharose 4 Fast Flow resin (GE Healthcare) conjugated with GFP nanobody.
- Glutathione Sepharose 4B resin (Cytiva)
- GST-tagged PreScission Protease
- 1 mM Cy3-CoA (DMSO)
- 1 mM LD655-CoA (DMSO)
- Sfp synthase
- AcpS
- Benzamidine
- Phenylmethylsulfonyl fluoride (PMSF)
- Soy trypsin inhibitor
- Aprotinin
- Leupeptin
- DNase I from bovine pancreas
- FreeStyle 293 media (GIBCO)
- Heat-inactivated fetal bovine serum
- 2 M sodium butyrate, filtered with a 0.22 µm filter
- bMRP1 P3 baculovirus
bMRP1 expression
bMRP1 P3 baculovirus is prepared as described by Goehring et al. 2014.bMRP1 constructs contain C-terminal PreScission-Protease-cleavable GFP tags.
- Culture HEK293S GnTI- suspension cells in FreeStyle 293 media supplemented with 2 % heat- inactivated fetal bovine serum, shaking at 37 ⁰C with 8 % CO2 and 80 % humidity.
- At a density of 2.5x106 cells/ml add 10 % (v/v) bMRP1 P3 baculovirus.
- After 18-20 hours add 10 mM sodium butyrate. Lower the temperature to 30 ⁰C.
- After additional 48 hours pellet the cells. Flash-freeze cells in liquid nitrogen and store at -80 ⁰C.
bMRP1 purification
All steps in this protocol should be carried out at 4 ⁰C, unless otherwise specified.
- Prepare resuspension buffer containing 300 mM NaCl, 50 mM Tris-HCl pH 8.0, 2 mM MgCl2, 2 mM DTT, 20 % glycerol, 1 μg/mL pepstatin A, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 100 μg/mL soy trypsin inhibitor, 0.6 mM benzamidine, 1 mM PMSF, and 3 µg/ml DNase I.
- Resuspend thawed cells in resuspension buffer using a handheld homogenizer (Polytron PT 1200E). Use 25 ml resuspension buffer per liter cell culture.
- Supplement with 2 % (w/v) LMNG and 0.2 % (w/v) CHS to solubilize membranes. Incubate 90 minutes, nutating at 4 ⁰C.
- Clarify the lysate by centrifugation at 75.000 g for 40 minutes at 4 ⁰C.
- Equilibrate NHS-activated Sepharose 4 Fast Flow resin (GE Healthcare) conjugated with GFP nanobody with 20 column volumes resuspension buffer. Use 2 ml resin bed volume per liter of cell culture.
- Mix the clarified lysate with GFP nanobody resin. Incubate 90 minutes, nutating at 4 ⁰C.
- Pack the slurry into a chromatography column.
- Wash the resin with 20 columnvolumes of wash buffer containing 0.06% digitonin, 150 mM NaCl, 50 mM Tris pH 8.0, 2 mM MgCl2, 2 mM DTT, and 5 % glycerol.
- Resuspend the resin in one column volume wash buffer. Add 0.35 mg/ml PreScission Protease. Incubate 2 hours at 4 ⁰C to cleave off the GFP tag.
- Equilibrate 1 ml bed volume Glutathione Sepharose 4B resin with 20 column volumeswash buffer.
- Collect the eluate from the GFP-nanobody resin by drippingthrough the Glutathione Sepharose 4B resin to remove PreScission Protease. Chase with two column volumes wash buffer.
- Concentrate the eluate to 500 µl using a 100 kDa MWCO Amicon Ultra centrifugal filter (Millipore).
- For ATPase measurements or electron cryomicroscopy continue with sectionNon-labeled samples. For fluorescent labeling of bMRP1 continue with section Site-specific labeling.
Non-labeled samples
- Attach a Superose 6 10/300 GL column (GE Healthcare) to a GE Amersham AKTA FPLC system with an in-line absorbance detector. Perform chromatography at 4 ⁰C.
- Equilibrate the chromatography column,with two column volumes size-exclusion chromatography buffer containing 0.06 % (w/v) digitonin, 150 mM KCl, 50 mM Tris-HCl pH 8.0, 2 mM MgCl2, and 2 mM DTT.
- Inject bMRP1 onto the column. Run the system at 0.4 ml/min, collecting 0.3 ml fractions.
- Pool and concentrate peak fractions to the desired concentration using a 100 kDa MWCO Amicon Ultra centrifugal filter (Millipore).
- Use the sample immediately or snap-freeze in liquid nitrogen and store at -80 ⁰C.
Site-specific labeling
The bMRP1 constructs used for site-specific labelling contain a 12-residue S6 peptide (GDSLSWLLRLLN) substituted at linker residues 868–879, and a 12-residue A1 peptide (GDSLDMLEWSLM) added to the C-terminus immediately following V1530. Sfp and AcpS synthases, as well as dye-CoA conjugates are prepared as described by Yin et al., 2006 and Zhou et al., 2007.
- To label the A1 site, add 5 µM AcpS, 25 µM LD655-CoA, and 10 mM MgCl2 to the concentrated eluate. Incubate 1 hour at room temperature.
- Remove AcpS and excess dye by size-exclusion chromatography as described in steps 1-3 of the section Non-labeled samples (Figure A).
- Concentrate peak fractions to 500 µl using a 100 kDa MWCO Amicon Ultra centrifugal filter (Millipore).
- To label the S6 site, add 5 µM Sfp synthase, 25 µM Cy3-CoA,and 10 mM MgCl2. Incubate1 hour at room temperature.
- Remove Sfp and excess dye by size-exclusion chromatography as described in points 1-3 of the section Non-labeled samples (Figure B).
- Concentrate bMRP1 to 10 µM using a 100 kDa MWCO Amicon Ultracentrifugal filter (Millipore).
- Aliquot and snap-freeze bMRP1 in liquid nitrogen. Store at -80 ⁰C.
- Analytical size-exclusion chromatography and SDS-PAGE may be performed to assess the homogeneity of the sample and the specificity of labeling (Figures C and D).
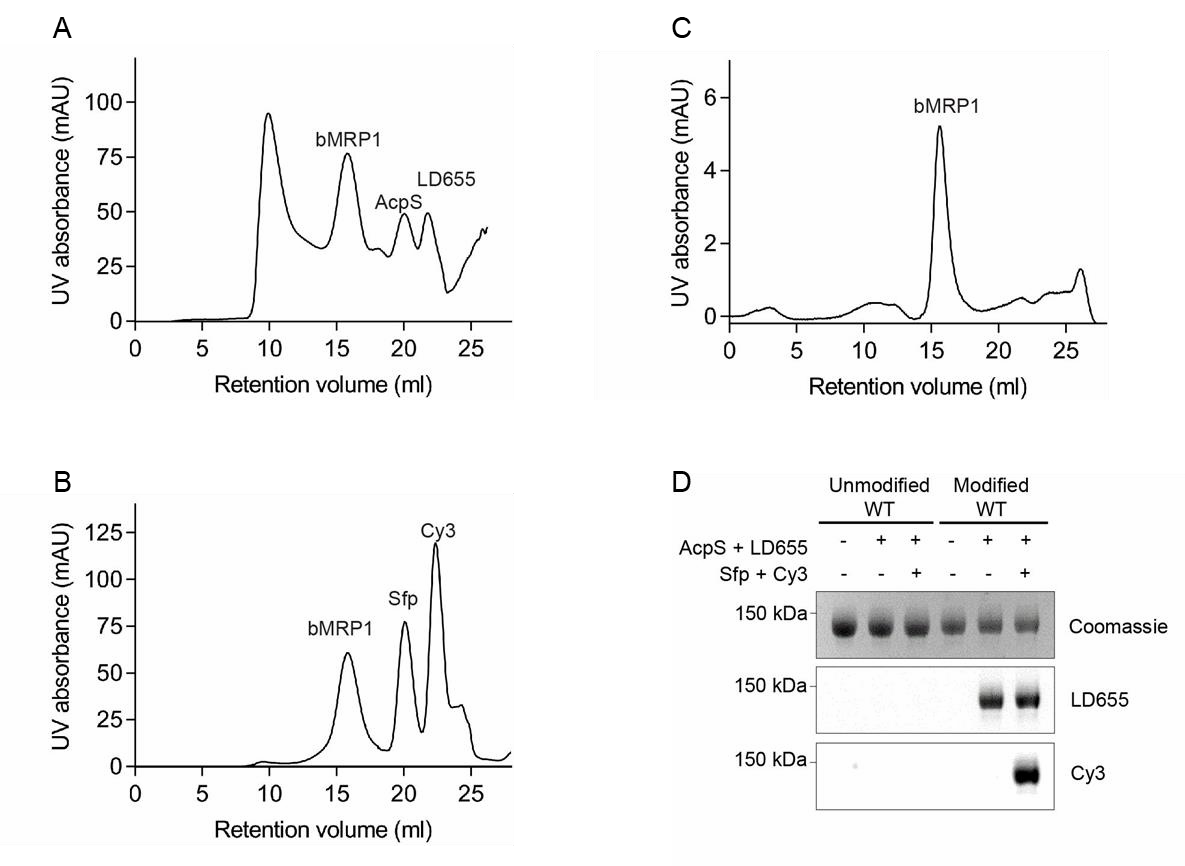
Purification and site-specific labeling of bMRP1
- Size-exclusion chromatography profile of bMRP1 after AcpS-catalysed labeling of the A1 site with LD655. UV absorbance was measured at 280nm.
- Size-exclusion chromatography profile of bMRP1 after Sfp-catalysed labeling of the S6 site with Cy3. UV absorbance was measured at 280 nm.
- Analytical Size-exclusion chromatography profile of bMRP1 after dual-labeling and removal of excess dye as well as AcpS and Sfp synthases. UV absorbance was measured at 280 nm.
- SDS-PAGE gel showing fluorescent labeling of bMRP1 with Sfp/AcpS synthases and dye-CoA conjugates. Modified WT stands for MRP1 with inserted peptide sequences for site-specific labeling.
Goehring A, Lee CH, Wang KH, Michel JC, Claxton DP, Baconguis I, Althoff T, Fischer S, Garcia KC, Gouaux E. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nature Protocols 9: 2574-2585 (2014)
Wang L, Johnson ZL, Wasserman MR, Levring J, Chen J, Liu S. Characterization of the kinetic cycle of an ABC transporter by single-molecule and cryo-EM analyses. eLife 2020;9:e56451 (2020)
Yin J, Lin AJ, Golan DE, Walsh CT. Site-specific protein labeling by sfp phosphopantetheinyl transferase. Nature Protocols 1: 280–285 (2006)
Zhou Z, Cironi P, Lin AJ, Xu Y, Hrvatin S, Golan DE, Silver PA, Walsh CT, Yin J. Genetically encoded short peptide tags for orthogonal protein labeling by sfp and AcpS phosphopantetheinyl transferases. ACS Chemical Biology 2: 337–346 (2007)
- Levring, J(2021). Protein expression, purification, and site-specific labeling. Bio-protocol Preprint. bio-protocol.org/prep845.
- Wang, L., Johnson, Z. L., Wasserman, M. R., Levring, J., Chen, J. and Liu, S.(2020). Characterization of the kinetic cycle of an ABC transporter by single-molecule and cryo-EM analyses. eLife. DOI: 10.7554/eLife.56451
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
