Advanced Search
SUMOylation and in vitro SUMOylation assay
Last updated date: Feb 4, 2021 Views: 894 Forks: 0
Please see file attached for detailed protocol on SUMO4 protein purification.
The Purification of SUMO4 Protein
Step 1. The Cloning of Mature SUMO4-expressing plasmids
As immature SUMO4 which contained V and Y amino acids within C-terminal can hardly be conjugated to the targeted protein based on our preliminary experiments, we constructed mature SUMO4-expressing plasmid which has removed V and Y amino acids within C-terminal, resulting in the exposure of SUMOylation donor sequences TGG amino acids. The corresponding DNA and protein sequences are showed below:
>SUMO4-Mature DNA Sequence
ATGGCCAACG AAAAGCCCAC AGAAGAAGTC AAGACTGAGA ACAACAATCA TATTAATTTG AAGGTGGCGG GACAGGATGG TTCTGTGGTG CAGTTTAAGA TTAAGAGGCA GACACCACTT AGTAAACTAA TGAAAGCCTA TTGTGAACCA CGGGGATTGT CAATGAAGCA GATCAGATTC CGATTTGGTG GGCAACCAAT CAGTGGAACA GACAAACCTG CACAGTTGGA AATGGAAGAT GAAGATACAA TTGATGTGTT TCAACAGCCT ACGGGAGGTT GA
>SUMO4-Mature Protein Sequence
MANEKPTEEV KTENNNHINL KVAGQDGSVV QFKIKRQTPL SKLMKAYCEP RGLSMKQIRF RFGGQPISGTDKPAQLEMED EDTIDVFQQP TGG
The SUMO4 DNA sequence is cloned into pET28a vector by double digestion with Nco I and Xho I. The finished clone harbors a 6×His within N-terminal. The corresponding DNA sequence is showed below:
>pET28a-6His-SUMO4
CC ATG GCC
CAC CAT CAC CAT CAC CAT
| 1 | ATG | GCC | AAC | GAA | AAG | CCC | ACA | GAA | GAA | GTC |
| 31 | AAG | ACT | GAG | AAC | AAC | AAT | CAT | ATT | AAT | TTG |
| 61 | AAG | GTG | GCG | GGA | CAG | GAT | GGT | TCT | GTG | GTG |
| 91 | CAG | TTT | AAG | ATT | AAG | AGG | CAG | ACA | CCA | CTT |
| 121 | AGT | AAA | CTA | ATG | AAA | GCC | TAT | TGT | GAA | CCA |
| 151 | CGG | GGA | TTG | TCA | ATG | AAG | CAG | ATC | AGA | TTC |
| 181 | CGA | TTT | GGT | GGG | CAA | CCA | ATC | AGT | GGA | ACA |
| 211 | GAC | AAA | CCT | GCA | CAG | TTG | GAA | ATG | GAA | GAT |
| 241 | GAA | GAT | ACA | ATT | GAT | GTG | TTT | CAA | CAG | CCT |
| 271 | ACG | GGA | GGT | TAA |
CTCGAG
Yellow-background nucleotides indicate restriction enzyme sites. Red nucleotides indicate 6His.
Green nucleotides indicate mature SUMO4 sequence.
Step 2. The expression test
The SUMO4-expressing plasmids are transformed into BL21 (Takara). Single clone is amplified in LB with kanamycin at 37℃ while shaking. When the OD600 of bacterial solution reached 0.4 to 0.6, the solution is transferred to 16℃ and added with isopropyl β-D-1-thiogalactopyranoside (IPTG) (Takara) to induce the expression of target protein. Another 18 hours later, protein-expressing bacteria are harvested by centrifuging and washed once with PBS. The resuspended bacteria are added with loading buffer and boiled directly at 100℃ for 10 min. Proteins within samples are separated by SDS- PAGE, followed by Coomassie blue staining. Both unstimulated and IPTG-stimulated samples are stained to distinguish induced proteins which are SUMO4. The typical Coomassie blue staining result is showed below (Figure 1):
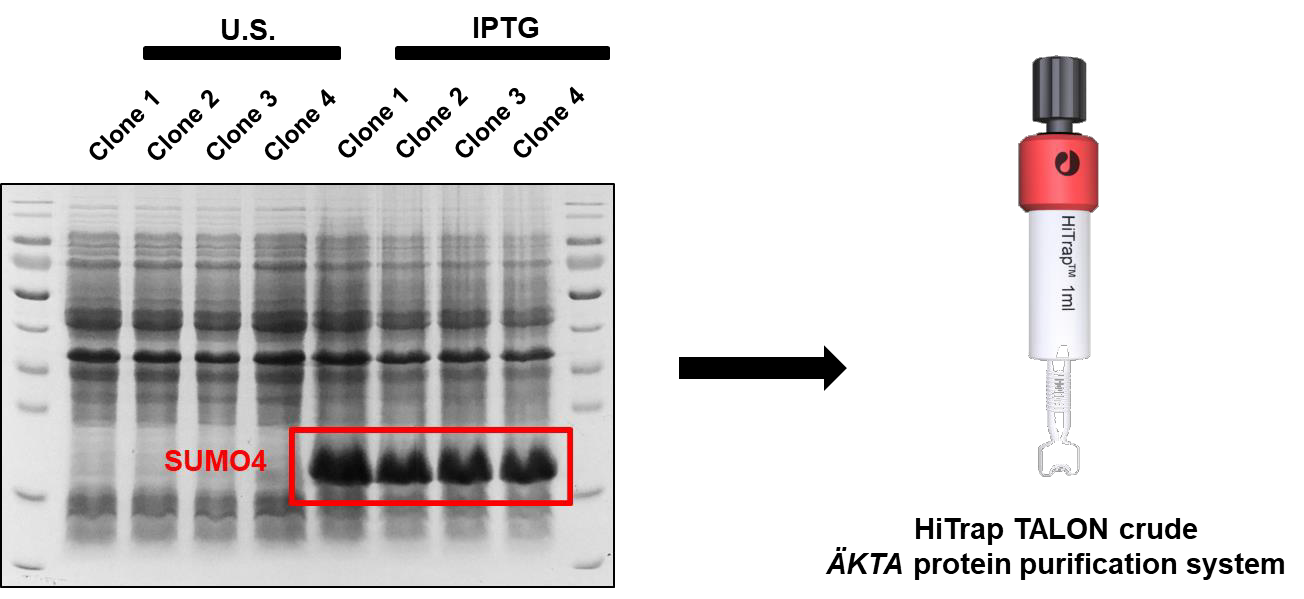
Figure 1. Coomassie blue staining result of unstimulated and IPTG-induced SUMO4-expressing bacteria.
Step 3. Searching for best elution condition
After having confirming the expression of SUMO4, the SUMO4-expressing bacteria lysates are proceeded to large scale protein purification which utilizes ÄKTA protein purification system(Figure 1). In orderto increase the purity of purified proteins,Co2+- containing column (HiTrap TALON Crude, Cytiva) is used to purify SUMO4 proteins instead of Ni2+ column. Proteins which are bound by Co2+ beads are linearly eluted by imidazole. The concentrations of imidazole are linearly increased from 0 mM to 500 mM. The SUMO4 proteins are eluted in the presence of 80 mM to 225 mM imidazole (16% to 45% of 500 mM imidazole solution) (Figure 2).
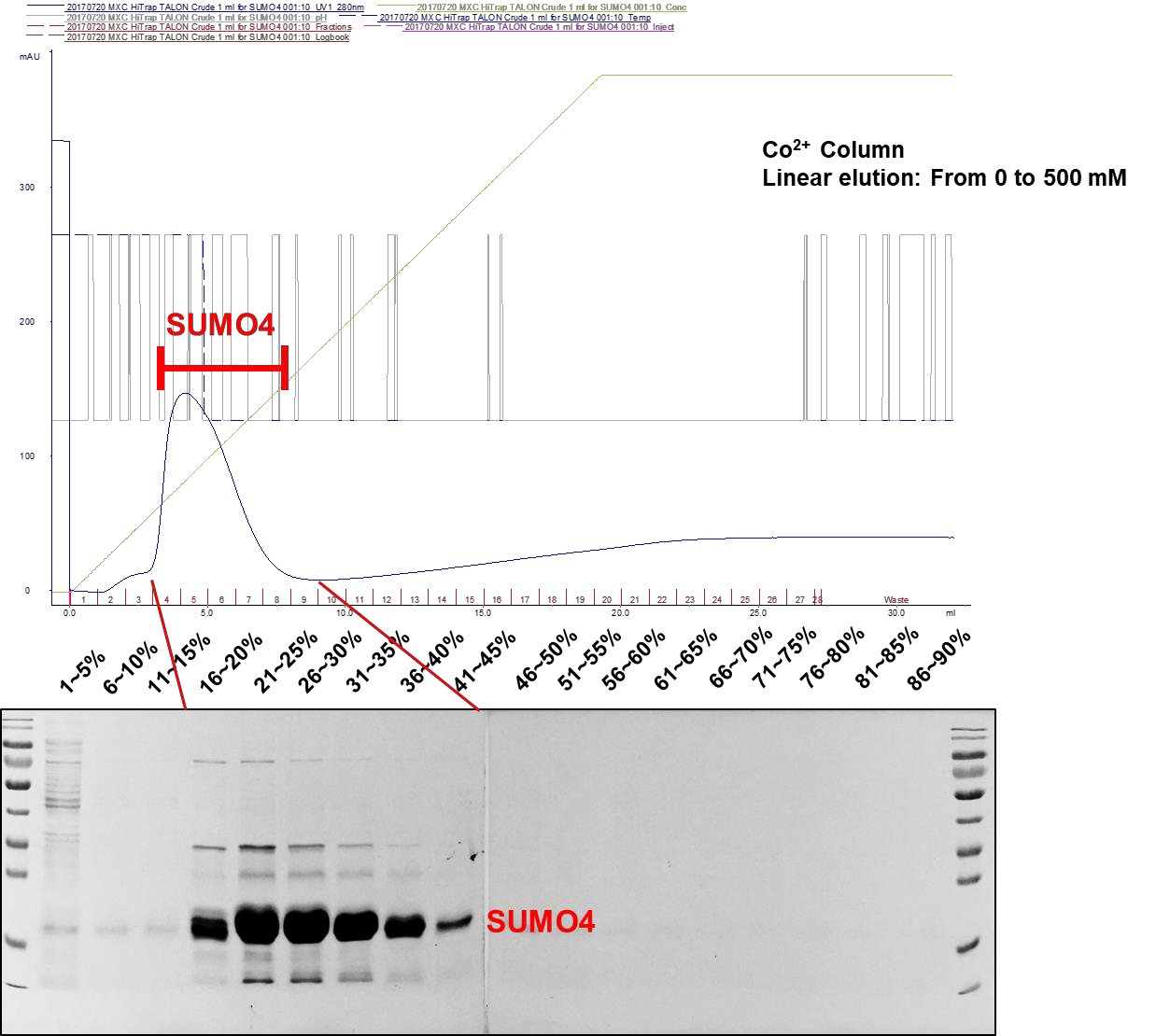
Figure 2. Linear elution of SUMO4 proteins. The peak indicates the SUMO4 protein retentions which are eluted in 16% to 45% of 500 mM imidazole (80 mM to 225 mM imidazole).
Step 4. Final elution of SUMO4 proteins
After determining the best ranges of imidazole concentrations to elute SUMO4 proteins, another large scale of SUMO4-expressing bacteria lysates are eluted for SUMO4 protein in gradient elution strategy. Firstly, 6His-SUMO4 proteins are bound to Co2+ column, followed by washing with 5 mM imidazole. The column is treated with 150 mM imidazole to elute SUMO4 proteins. After eluting for SUMO4 proteins, the column is wash again with 500 mM imidazole to remove any residual proteins (Figure 3). The column is kept in 20% ethanol. The 150 mM imidazole-eluted SUMO4 proteins are concentrated and buffer-exchanged to PBS with 3 kD ultrafiltration device. The concentration is determined by BCA assay. The prepared SUMO4 proteins can be used to conduct in vitro SUMOylation assays, or stored at -80℃.
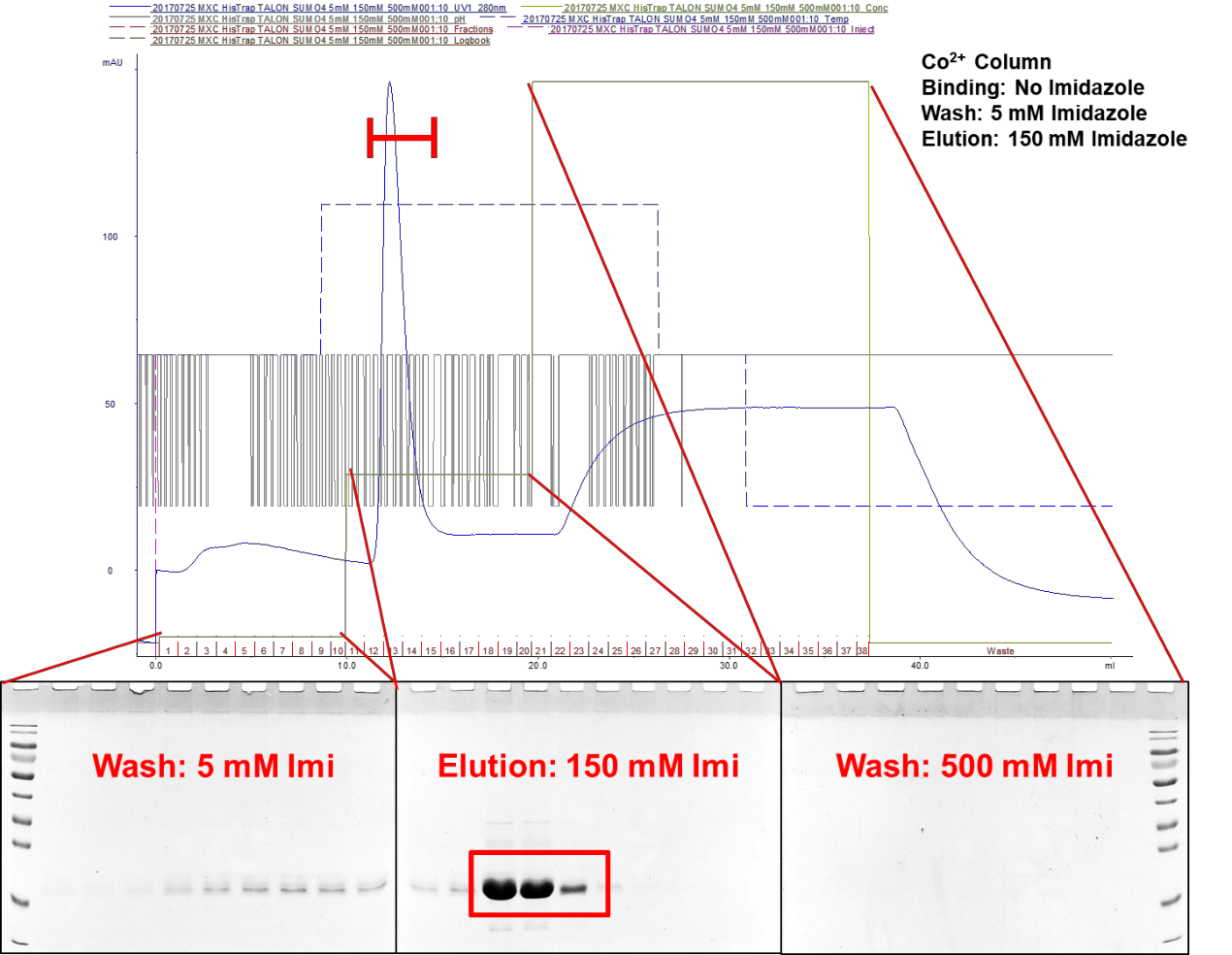
Figure 3. Gradient elution strategy to harvest pure SUMO4 proteins. SUMO4 proteins are washed with 5 mM imidazole from the Co2+ column which are bound with 6His-SUMO4 proteins. SUMO4 proteins are eluted with 150 mM imidazole and concentrated with 3 kD ultrafiltration device.
- Ma, X(2021). SUMOylation and in vitro SUMOylation assay. Bio-protocol Preprint. bio-protocol.org/prep817.
- Ma, X., Yang, T., Luo, Y., Wu, L., Jiang, Y., Song, Z., Pan, T., Liu, B., Liu, G., Liu, J., Yu, F., He, Z., Zhang, W., Yang, J., Liang, L., Guan, Y., Zhang, X., Li, L., Cai, W., Tang, X., Gao, S., Deng, K. and Zhang, H.(2019). TRIM28 promotes HIV-1 latency by SUMOylating CDK9 and inhibiting P-TEFb. eLife. DOI: 10.7554/eLife.42426
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
