In vitro Anti-tumor TIL expansion experiment
Sri Krishna and Frank J Lowery, Surgery Branch, NCI, 01/08/2021
Goal: To identify anti-tumor ecognition and total cell yield upon repeated expansions from different TIL subsets.
- Thaw tumor infiltrating T cells (TILs) from infusion product and rest overnight in a T25 flask in 10mL of TIL-media without any cytokines
- TIL subsets from the infusion product (CD8+ or CD39- CD69- or CD39+ CD69+) were isolated (5e5 total cells per subset) by flow cytometry-based cell sorting.
Note: Sort for more than 5e5 cells to account for sample loss.
- Rest flow sorted TIL subsets for an hour without cytokines
- Harvest and count autologous patient target tumor cell line
- Set up tumor-TIL co culture by co-incubating 1e5 TIL subset with 1e5 tumor cells per well in a 96 well plate in 100uL TIL-media without cytokines overnight (5 wells each for each condition).
- Next day, use 41BB (CD137) based sorting to isolate tumor-reactive flow-sorted TIL subset (41BB+).
- 1e5 tumor-reactive 41BB+ cells are sorted for each TIL-subset that has been cocultured (e.g. CD8+ 41BB+ or CD8+ CD39- CD69- 41BB+ or CD8+ CD39+ CD69+ 41BB+).
Note: Sort for more than 1e5 cells to account for sample loss.
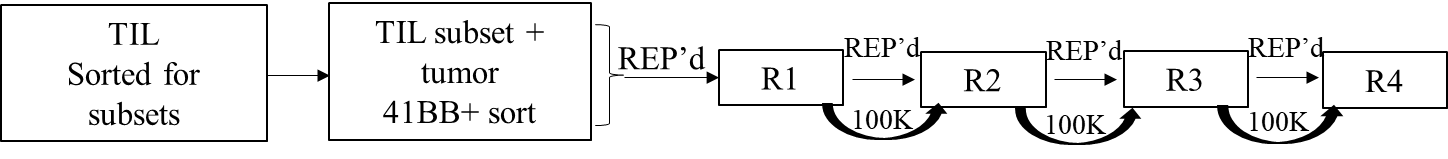
- Perform rapid expansion protocol (REP): each 1e5 sorted 41BB+ TIL subsets with 2e7 irradiated allogeneic feeders (50 Gy), 30ng/mL OKT3 antibody (anti-CD3), and 3000 U IL-2 in a T25 flask (day 0) in TIL-media.
Note: Have a T25 control flask with irradiated feeders alone to ensure feeders do not outgrow.
- Check for expansion every 2-3 days. On day 5 if activated cell clusters are apparent initiate media change using 6000U IL-2. Note: Do not add OKT3 from now on.
- Continue media change and TIL expansion into T75 or bigger flasks if needed for two weeks from starting REP.
- Count cells and set up autologous tumor coculture as in step #5. Readout can be done on IFNg ELISpot or 41BB upregulation overnight. Or cells can be frozen to be assayed for tumor-recognition later.
- Same day, set up second round of re-expansion if needed using 1e5 TILs from REP1 and 2e7 irradiated allogeneic feeders as shown in step #8.
- Follow steps 8-11, if needed.
- If subsequent TIL REPs are needed to be assayed for anti-tumor recognition, freeze all samples down and perform anti-tumor TIL recognition together.
- Count total cell yield at each step from each REP. Calculate the theoretical cell yield from normalizing to total cell yield from each REP step.
Antibodies:
Anti-CD8-PE-Cy7 (RPA-T8, 1:300, BD Biosciences, USA)
Anti-CD39-FITC (A1; 1:200, BioLegend, USA)
Anti-CD69-APC (FN50; 1:25, BD Biosciences, USA)
Anti-CD137-PE (4B4-1, BD Bisociences, USA)
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this
article to respond.

