Advanced Search
STED imaging
Last updated date: Dec 10, 2020 Views: 908 Forks: 0
Immunofluorescence staining for STED microscopy
1. Fix DIV8 hippocampal neurons (plated on 13 mm diameter precision cover glasses; thickness 1.5H), with 500 ml 4% PFA for 20 minutes at room temperature (RT)
2. Remove PFA and wash briefly with PBS
3. Incubate coverslips with 0.1% Triton X-100 (in PBS) for 3 minutes at RT
4. Wash briefly with PBS
5. Incubate 3 minutes with 0.2M NH4Cl (in H2O)
6. Wash briefly with PBS
7. Block the coverslips using 5% FBS in PBS (blocking buffer), for 60 minutes at RT
8. Incubate the primary antibodies at the appropriate dilution, prepared in blocking buffer, overnight at 4ºC
9. Wash the coverslips with PBS (3 x 5 minutes each wash)
10. Incubate with secondary antibodies (and phalloidin if needed), prepared in blocking buffer, 60 minutes at RT
11. Wash the coverslips with PBS (3 x 5 minutes each wash)
12. Mount coverslips in adhesion histobond slides using mounting media (90% glycerol, 0.5 N-propyl gallate, 200 mM Tris pH8.0).
STED imaging on an inverted Leica TCS SP8 STED 3X (Leica Microsystems)
1. Place slides (inverted) on the 100x NA 1.4 STED WHITE oil immersion objective
2. Identify axons as bearing an axon initial segment (ankyrinG-positive or pMLC-positive) or as MAP2-negative (488 nm channel), using the confocal mode
3. Measure the distance from the cell body (we generally acquire images at 80-100 µm from the cell body) using the ruler tool of the Leica processing menu
4. Image using the STED mode; we generally image βII-spectrin in the 580 nm channel and NMIIA/B or phalloidin in the 633 channel
5. Use the following STED settings:
- Lateral resolution enhancement with 20 nm pixel size in xy and dwell times of 600 ns
- STED far-red channel (Abberior STAR 635P) recorded with 633 nm excitation using the pulsed white light laser with 80 MHz repetition rate; STED depletion with a synchronized pulsed 775 nm depletion laser; Detection bandpass set to 650 to 750 nm; Pinhole set to 0.93AU
- Second STED channel (Abberior STAR580) recorded in line sequential mode with 561 nm excitation and 775 nm depletion using a detection window from 580 to 620 nm.
- Apply the following acquisition settings: 16 x line averaging and detector gating on a hybrid detector (HyD, Leica Microsystems) of 0.3 ns to 6 ns. Field of view: use format 1024x1024 and a zoom factor of 6.
Analyses of axon diameter
1. Open images in ImageJ and select axon stretches unequivocally focused in the maximum wide plan
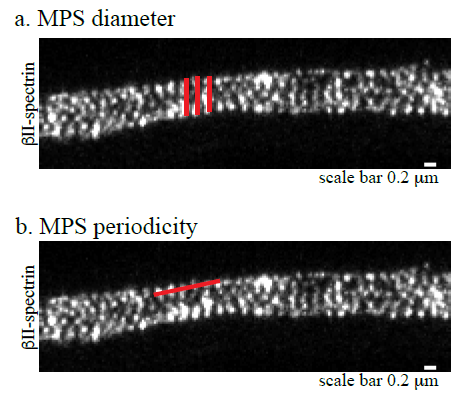
2. Use the straight line of the selection tool to measure the distance between the outer points of βII-spectrin or phalloidin staining (as depicted in red in the Figure1a)
3. Use the Measure tool to obtain measurements in the Analyze Menu.
Analysis of periodicity of βII-spectrin or phalloidin staining
1. Open images in ImageJ and draw a straight line, using the selection tool, spanning consecutive βII-spectrin or phalloidin stained rings in the outermost region of the axon (as shown in Figure 1b, red line)
2. Use the line profile tool, within the Analyze Menu, to obtain a graph where the x-axis represents the distance along the selected line and the y-axis the pixel intensity
3. Calculate the distance between the values corresponding to the maximum intensity
Antibodies and actin staining:
Antibody | Dilution | Company | Catalogue |
Rabbit polyclonal NMIIA | 1/200 | Sigma-Aldrich | M8064 |
Rabbit polyclonal NMIIB | 1/200 | Sigma-Aldrich | M7939 |
Mouse monoclonal βII-spectrin | 1/200 | BD Transduction | 612563 |
Rabbit polyclonal MAP2 | 1/10000 | Synaptic Systems | 188002 |
Rabbit polyclonal AnkyrinG | 1/200 | Synaptic Systems | 368003 |
Rabbit polyclonal 2xphospho MLC | 1/200 | Cell Signaling | 3674 |
Anti-rabbit STAR 635 | 1/200 | Abberior GmbH | 2-0012-007-288 |
Anti-mouse STAR580 | 1/200 | Abberior GmbH | 2-0012-005-8 |
Anti-rabbit AF488 | 1/500 | Jackson Immunoresearch | 711-545-152 |
Phalloidin 635P | 1/100 | Abberior GmbH | 2-0205-002-5 |
- Sousa, M(2020). STED imaging. Bio-protocol Preprint. bio-protocol.org/prep697.
- Costa, A. R., Sousa, S. C., Pinto-Costa, R., Mateus, J. C., Lopes, C. D., Costa, A. C., Rosa, D., Machado, D., Pajuelo, L., Wang, X., Zhou, F., Pereira, A. J., Sampaio, P., Rubinstein, B. Y., Mendes Pinto, I., Lampe, M., Aguiar, P. and Sousa, M. M.(2020). The membrane periodic skeleton is an actomyosin network that regulates axonal diameter and conduction. eLife. DOI: 10.7554/eLife.55471
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
