Advanced Search
Adoptive cell transfer (ACT) and RNA-LPX treatment
Last updated date: Dec 9, 2020 Views: 1057 Forks: 0
Day of adoptive cell transfer (ACT)
Irradiate tumor bearing or non-tumor bearing WT mice 4 h prior ACT with sublethal dose of 2.5-5 Gy using an XRAD320 irradiator and a dose range of 0.44 Gy/min. No irradiation is needed for NSG mice.
ACT of murine T cells (alternative I):
Harvest murine transduced T cells from cell culture vessels into appropriate tubes.
Wash T cells with murine T cell medium once.
Layer carefully 5 volumes transduced T cells to 3 volumes Ficoll-Paque PREMIUM (1.084 g/mL).
Perform density gradient centrifugation with reduced acceleration and strongly reduced break for 20 min, 800 ×g to remove debris.
Harvest cells in lymphocyte layer and wash cells twice with PBS to remove remaining serum proteins.
Determine cell count of total viable cells.
Take 0.2-0.5 ×106 cells for transgene (CAR or GFP) expression analysis by flow cytometry.
Calculate the total amount of transgene (CAR or GFP) positive cells in total viable cells.
Adjust T cell numbers to transgene positive T cells/200 µL with PBS (e.g. 1 ×106 CAR expressing T cells/200 µL/ mouse).
Inject 200 µL of cell solution carefully into the retrobulbar venous plexus of anesthetized WT mice (e.g. by 2.5 % Isofluran inhalation anesthesia) with 30 G syringe (e.g. BD Micro-Fine).
ACT of human T cells (alternative II):
Harvest human transduced T cells directly from cell culture vessels into appropriate tubes after transduction process. Alternatively thaw frozen transduced T cells at 37°C and transfer cells into 10 volumes of human T cell medium supplemented with 2 ng/mL DNAseI and invert carefully.
Wash cells twice with PBS to remove remaining serum proteins.
Determine cell count of total viable cells.
Take approximately 0.2-0.5 ×106 cells for transgene (CAR surface) expression analysis by flow cytometry.
Calculate the total amount of transgene (CAR or GFP) positive cells in total viable cells.
Adjust T cell numbers to transgene positive T cells/200 µL with PBS (e.g. 1 ×106 CAR expressing T cells/ 200 µL/ mouse).
Inject 200 µL of cell solution carefully intravenously into the retrobulbar venous plexus of anesthetized NSG (e.g. by 2.5 % Isofluran inhalation anesthesia) with 30 G syringe.
Day of RNA-Lipoplex (RNA-LPX) treatment
Work under RNase-free conditions.
Bring the following stock solutions to room temperature: in vitro transcribed RNA (refer to RNA), nuclease-free water, 1.5 M sodium chloride and liposomes. F12 Liposomes contain 66.6 mol cationic DOTMA per 33.3 mol helper lipid DOPE in a ratio 2:1 – according to WO/2013/143555 and Kranz et al. 2016.
Calculate needed volumes of RNA, sodium chloride, liposomes and water for complexation. Thereby consider a charge ratio of 1.3 to 2 of cationic DOTMA and RNA for lipoplex mediated delivery into secondary lymphoid organs. The final concentration of sodium chloride should be 150 mM in generated RNA-LPXs.
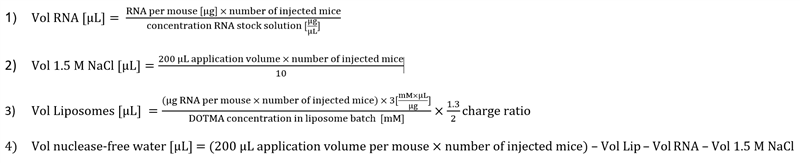
Mix thoroughly calculated volumes of nuclease-free water, 1.5 M sodium chloride, 20-40 µg RNA (encoding for e.g. tumor-antigen or T cell epitopes) in a nuclease-free tube. Add liposomes at last and vortex immediately for 10 seconds.
Incubate generated RNA-LPX 10 min at room temperature.
Inject RNA-LPX carefully intravenously into the retrobulbar venous plexus of anesthetized NSG (e.g. by 2.5 % Isofluran inhalation anesthesia) with 30 G syringe.
Solutions:
Murine T cell medium
RPMI1640-GlutaMAX supplemented with 10% (v/v) heat-inactivated FBS, 1x non-essetial amino acids, 1 mM sodium pyruvate, 10 mM HEPES, 50 μM β-Mercaptoethanol, 50 IU/mL Penicillin and 50 μg/mL Streptomycin.
Human T cell medium
X-VIVO 15 medium (Lonza) supplemented with 5% (v/v) human serum.
1.5 M NaCl solution
Dilute 1 mL sterile and RNase-free 5 M sodium chloride solution from e.g. Ambion with 2.33 mL nuclease-free water. Scale-up accordingly.
Reference
Kranz, Lena M.; Diken, Mustafa; Haas, Heinrich; Kreiter, Sebastian; Loquai, Carmen; Reuter, Kerstin C. et al. (2016): Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. In: Nature 534 (7607), S. 396–401. DOI: 10.1038/nature18300.
- Reinhard, K(2020). Adoptive cell transfer (ACT) and RNA-LPX treatment. Bio-protocol Preprint. bio-protocol.org/prep695.
- Reinhard, K., Rengstl, B., Oehm, P., Michel, K., Billmeier, A., Hayduk, N., Klein, O., Kuna, K., Ouchan, Y., Wöll, S., Christ, E., Weber, D., Suchan, M., Bukur, T., Birtel, M., Jahndel, V., Mroz, K., Hobohm, K., Kranz, L., Diken, M., Kühlcke, K., Türeci, �., Sahin, U., Reinhard, K., Rengstl, B., Oehm, P., Michel, K., Billmeier, A., Hayduk, N., Klein, O., Kuna, K., Ouchan, Y., Wöll, S., Christ, E., Weber, D., Suchan, M., Bukur, T., Birtel, M., Jahndel, V., Mroz, K., Hobohm, K., Kranz, L., Diken, M., Kühlcke, K., Türeci, �. and Sahin, U.(2020). An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors . Science 367(6473). DOI: 10.1126/science.aay5967
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
