Protocol – Live Cell Imaging of EGF Binding to A431 Cells
Reagents
- Tetramethylrhodamine-labeled recombinant EGF (ThermoScientific)
- Imaging media: 117 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 20 mM HEPES, Glucose, BSA
- NaCl: 6.83 hg/L
- KCl: 0.395 g/L
- HEPES: 4.77 g/L
- NaH2PO4-2H2O: 0.195 g/L
- Glucose: 9g/L
- BSA: 100mg/L (add after autoclave)
- Adjust PH to 7.2 after autoclave
- A431 cell (ATCC)
Equipment
- Interchangeable coverglass dish (ICD) with lid (Bioptechs Inc)
- 30 mm round coverglass (Bioptechs Inc)
- Stable-Z temperature-controlled microscopy stage (Bioptech Inc)
- Objective heater (Bioptech Inc)
- HILO fluorescence microscope (home-build using Olympus IX frame)
- Standard fluorescence filter set for TMR (Semrock Inc)
- Plasma cleaner (Harrick Plasma)
Procedure
- Culture A431 cells according to the ATCC protocol (DMEM medium supplemented with 10% FBS).
- Two days before imaging experiment: clean/sterilize 30-mm coverglass with an O2 plasma cleaner (medium power, 3min).
- Seed A431 cells on coverglass at low density (~10,000 cell per coverglass) according to the standard sub-culture protocol. Culture normally for 24 hours.
- One day before the imaging experiment: replace culture medium with serum-free DMEM medium. Culture overnight.
- On the day of imaging experiment: Set the temperatures of the microscopy stage and objective heater to 37 °C.
- Prepare imaging media and TMR-EGF-containing imaging media to 2x of the desired concentration, e.g., for final concentration of 25 ng/ml EGF, prepare imaging media with 50 ng/ml TMR-EGF. Pre-warm all imaging media to 37 °C.
- Assemble imaging chamber using ICD and the glass coverglass. Add 1ml pre-warmed EGF-free imaging medium before covering with lid. Install the imaging chamber on the microscope to allow the temperature to re-equilibrate.
- Using cell auto-fluorescence as a cue, adjust HILO excitation angle to be minimal yet still allow whole-cell illumination.
- Reduce field-aperture to be as small as possible, but allowing single-cell imaging.
- Engage autofocus.
- Start time-lapse imaging at 0.1-0.2 Hz imaging speed. While recording images, using a syringe, add 1-ml pre-warmed EGF-containing imaging medium via the perfusion port of the ICD lid into the imaging chamber.
- Fluorescence signal at the cell should quickly rise and typically reach the saturation level within minutes. A typical result is shown below.
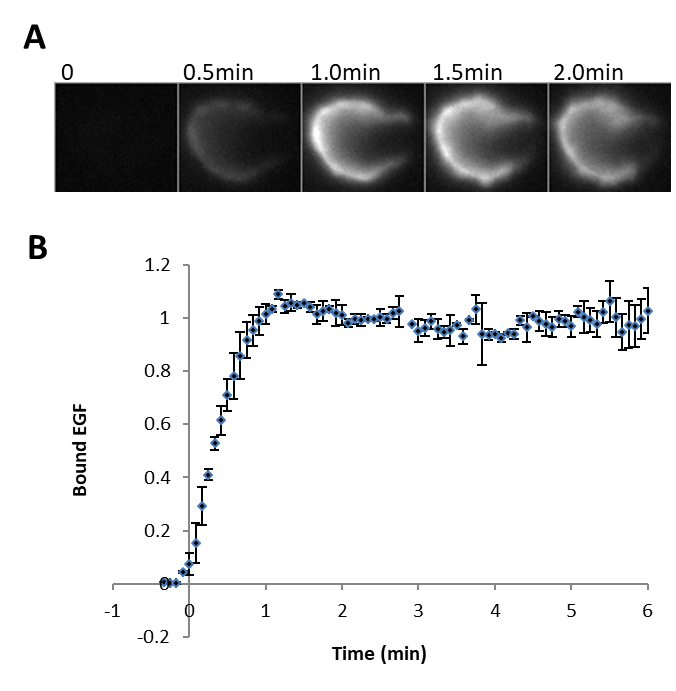
Copyright: Content may be subjected to copyright.
How to cite:Readers should cite both the Bio-protocol preprint and the original research article where this protocol was used:
- Oh, D, Jadwin, J, Mayer, B and Yu, J(2020). Live Cell Imaging of EGF Binding to A431 Cells. Bio-protocol Preprint. bio-protocol.org/prep595.
- Jadwin, J. A., Oh, D., Curran, T. G., Ogiue-Ikeda, M., Jia, L., White, F. M., Machida, K., Yu, J. and Mayer, B. J.(2016). Time-resolved multimodal analysis of Src Homology 2 (SH2) domain binding in signaling by receptor tyrosine kinases. eLife. DOI: 10.7554/eLife.11835
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this
article to respond.
 Post a Question
Post a Question0 Q&A

