Isolation of synaptosomes:
- Prepare six Ficoll gradients (one gradient for each rat). From bottom to top: 4 mL 13%, 1 mL 9% and 4 mL 6% Ficoll solution
- Decapitate six 5-6-week-old rats, excise the brains, remove the white matter as much as possible and homogenize the brains in 30 mL of ice-cold homogenization buffer in a glass-teflon homogenizer (add 30 µl of PMSF and 30 µl of pepstatin A stock solutions) – perform 9 strokes at 900 rpm.
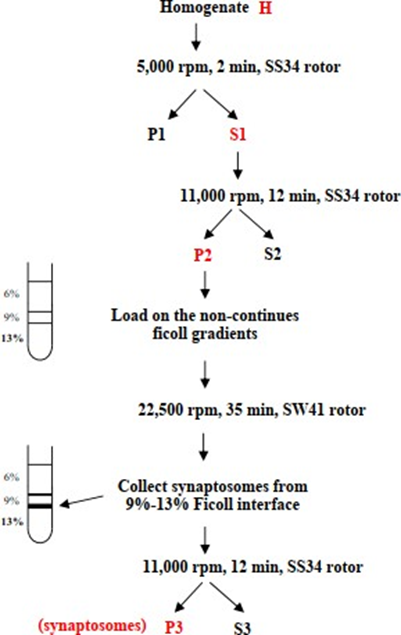
- Centrifuge the brain homogenate at 5000 rpm for 2 min using a SS34 rotor (Sorvall centrifuge) to pellet nuclei and cell debris.
- Collect the supernatant S1 and discard the pellet P1. Centrifuge S1 at 11,000 rpm for 12 min (SS34 rotor, Sorvall).
- Re-suspend the pellet P2, avoiding the dark brown part containing mitochondria) in 18 mL of homogenization buffer.
- Load 3 mL each of the re-suspended pellet onto the Ficoll gradient using a pipette fitted with a tip of which the end is cut off to avoid any damage to synaptosomes by shearing forces.
- Centrifuge the gradients using SW41 rotor (Beckmann centrifuge) at 22,500 rpm for 35 min.
- Collect the synaptosomes, enriched in the 9-13 % Ficoll interface, using a Pasteur pipette and transfer them into a fresh SS34 tube for the next washing step.
Add 30 mL of homogenization buffer and centrifuge the suspension at 11,000 rom for 12 min, SS34 rotor. Re-suspend the resulting pellet P3 in 2.5 mL of homogenization buffer/rat brain, supplemented with PMSF and pepstatin A inhibitors. Keep the synaptosomes on ice until use.
Glutamate release assay:
- Centrifuge 1 mg of synaptosomal protein at 8500 for 2 min, room temperature (the supernatant will be not cleared, to keep synaptosomes intact we do not increase the speed of centrifugation)
- Very gently resuspend the pellet in 1 mL pre-warmed sodium buffer, again using a pipet with a blue tip of which the end is cut off
- Incubate for5 min at 37°C in a water bath
- Add 10 µl of NADP (final concentration: 1 mM) and 2.6 µl of CaCl2 (final concentration: 1.3 mM) or 5 µl of EGTA (final concentration: 0.5 mM)
- Transfer the sample in the glass cuvette using a cut blue tip and put the cuvette in the spectrofluorometer, set excitation wavelength to 340 nm and emission to 440 nm.
- Record thesignal for3 min to obtain a stable baseline.
- Add 50µl glutamate dehydrogenase (total 200 Units)
- Record for 3 more min.
- Stimulate synaptosomesby adding25 µl of 2 M KCl solution.
- Recordthe responsefor another2-5 min.
Homogenization Buffer
320 mM sucrose, 5mM HEPES, pH 7.4 Sterile filtered and degassed. Store at 4°C
Freshly add protease inhibitors: Pepstatin (Final concentration 1µg/ml) and PMSF (Final concentration 200 mM)
Ficoll Gradients
6%, 9% and 13% (w/v) Ficoll in Homogenization buffer
From bottom to top: 4 mL 13%, 1 mL 9% and 4 mL 6% Ficoll
Sodium Buffer:
140 mM NaCl, 5 mM KCl, 20mM HEPES, 5 mM NaHCO3, 1.2 mM Na2HPO4, 1 mM MgCl2, 10 mM glucose, pH 7.4
NADP: 100 mM
CaCl2: 0.5 mM
EGTA: 100 mM
KCl: 2 M
Glutamate dehydrogenase from bovine liver: 200 Unit/reaction
Synaptosome isolation from rat brain (overview):
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this
article to respond.

