- Culture 500,000 cells/ well with complete growth media in 6 well plate.
- Once the culture reaches 80-90% confluency, take 20 µl of the media from the well and mix it with the Tris-Glycine-SDS sample buffer (Thermo Fisher Scientific; LC2676). The amount of media loaded in the gel should be optimized because different cells will release a different amount of MMPs.
- Load the mixture of media and sample buffer in a 10% Novex Gelatin Zymogram Plus gel (Thermo Fisher Scientific; ZY00100). Not all the MMPs activity can be detected by gelatin zymogram because different MMPs react to different matrixes such as gelatin, casein, and collagen.
- Run the gel in 1X Tris-glycine SDS running buffer (Thermo Fisher Scientific; LC2675) till the loading dye exit the gel.
- Prepare 1X Novex Zymogram Renaturing buffer (Thermo Fisher Scientific; LC2670) by diluting it in 1:9 dilution with deionized water.
- After electrophoresis, keep the gel in 20 ml renaturing buffer for 30 min with continuous gentle shaking at room temperature.
- Prepare 1X Novex Zymogram developing buffer (Thermo Fisher Scientific; LC2671) by diluting it in 1:9 dilution with deionized water.
- Discard the renaturing buffer and add 20 ml developing buffer for 30 min with continuous gentle shaking at room temperature.
- Discard the developing buffer and add fresh 30 ml developing buffer and keep it at 37° incubator overnight (shaking is not required).
- Step 9 is crucial for optimization because activity and, therefore, the band intensity of MMPs depend on sample loading and incubation time in developing buffer.
- Stain the gel with Simply BlueTM Safe Stain (Thermo Fisher Scientific; LC6065) for 1 hr at room temperature with gentle shaking.
- Image/ scan the gel using an imager capable of imaging SDS-PAGE gel. The area of MMPs activity will appear as a clear band against dark background.
- MMPs can be distinguished based on the molecular weight of the area of activity of MMPs.
Quantify the intensity of the area of MMPs activity using ImageJ and normalize it to protein isolated from the cells from which the media was taken to perform zymography.
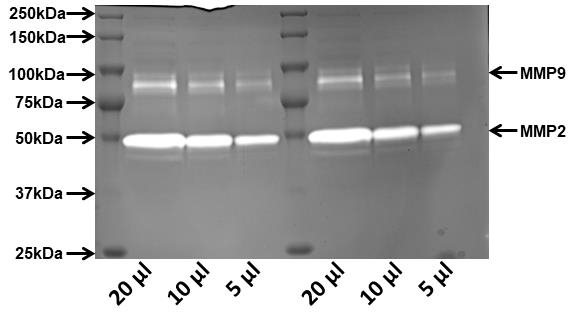
Figure 1: Image showing a gelatine zymogram gell where the light bands indicate the activity of MMP2 and 9 in a dose-dependent manner. The media was collected from the wells where HCT116 colorectal cancer cells were grown for 24 hr. The wells in the zymogram were loaded with 20 µl, 10 µl, and 5 µl of media.
Copyright: Content may be subjected to copyright.
How to cite:Readers should cite both the Bio-protocol preprint and the original research article where this protocol was used:
- Pathak, T(2020). Zymography. Bio-protocol Preprint. bio-protocol.org/prep557.
- Pathak, T., Gueguinou, M., Walter, V., Delierneux, C., Johnson, M. T., Zhang, X., Xin, P., Yoast, R. E., Emrich, S. M., Yochum, G. S., Sekler, I., Koltun, W. A., Gill, D. L., Hempel, N. and Trebak, M.(2020). Dichotomous role of the human mitochondrial Na+/Ca2+/Li+ exchanger NCLX in colorectal cancer growth and metastasis. eLife. DOI: 10.7554/eLife.59686
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this
article to respond.
 Post a Question
Post a Question0 Q&A
