Advanced Search
Reverse phase(RP)-high-performance liquid chromatography (HPLC) analysis of globin chains
Last updated date: Jul 28, 2020 Views: 1246 Forks: 0
RP-HPLC analysis allows the identification of globin chains composing the hemoglobin tetramers based on their different hydrophilic properties and the determination of the relative amount of globin chains. This protocol was designed for evaluating globin in (i) red blood cells (RBCs) from human whole blood and (ii) in vitro differentiated RBCs or burst-forming unit-erythroid (BFU-E).
Sample preparation
Lyphochek Hemoglobin A2 control (Biorad, ref: #553):
This product is prepared from human whole blood and contains different forms of hemoglobin (A, A2, F, and S). It is used to generate quality and elution control profiles for each globin chain.
Reconstitute the lyophilized product in 500 µl of deionized water and let it stand for 10 minutes, swirling occasionally.
Before preparing the aliquots, gently invert the vial several times to obtain an homogeneous solution.
Prepare aliquots of 5 µl of the solution mixed with 575 µl of deionized water in 1.5-mL microtubes.
Store aliquots at -80°C for long-term use.
Before each HPLC run, thaw an aliquot and dilute it 1:10 with ice-cold deionized water.
Put 50 µl of the diluted control in a HPLC loading tube.
Storage: The solution is stable at -80°C for long-term use and stable at 4°C for 7 days.
Whole blood:
In a 1.5-ml microtube, centrifuge 100 µl of blood 5 min @ 300 g.
Discard the supernatant.
Add 900 µl of ice-cold deionized water to the RBC pellet.
Vortex the samples in order to favor RBC lysis.
Centrifuge 10 min @ 9,500 g 4°C.
Collect the supernatant and dilute it 1:100 in ice-cold deionized water.
Put 10 µl of the diluted sample and 30 µl of ice-cold deionized water in a HPLC loading tube.
Storage: RBC pellets can be stored at 4°C for 7 days for short-term use or at -80°C for long-term use.
In vitro differentiated RBCs (500,000 cells) and BFU-E (approximately 25 colonies):
In a 1.5 ml microtube, resuspend the cell pellet in 50 µl of ice-cold deionized water.
Vortex the samples in order to favor RBC lysis.
Centrifuge 10 min @ 9,500g 4°C.
Put 40 µl of the lysate in a HPLC loading tube.
Storage: Lysed differentiated RBCs or BFU-E can be stored at 4°C for 7 days for short-term use or at -80°C for long-term use.
MATERIALS
Instrumentation and chromatography
The analysis is performed using a NexeraX2 SIL-30AC chromatograph (Shimadzu) composed by:
Detector unit (SPD-20AV) measuring the absorbance of each globin chain at λ=220nm.
Degassing unit (DGU-20A3R) removing air bubbles from the mobile phase during the acquisition.
Solvent distribution unit (LC-30AD) composed by 2 pumps delivering mobile phase to the column. Note: the pressure does not have to exceed 200bar during the acquisition.
Injection unit (SIL-30AC) injecting samples (35 µl) into the mobile phase.
Columns hoven (CTO-20A) heating the column of interest at 40°C for a better resolution and reproducibility of the analysis between different runs. Samples are injected in a RP-column specific for proteins with a mass higher than 10kDa; in this protocol we use the Aeris™ 3.6µm WIDEPORE C4 200 Å, LC Column 250 x 4.6 mm (Phenomenex)
Acquisition and analysis are performed using the LC Solution software.
Chemicals and reagents
Mobile phases are composed by HPLC-grade acetonitrile (ACN; Sigma-Aldrich), creating a hydrophobic environment and Trifluoroacetic acid (TFA; Sigma-Aldrich) that breaks the heme group within the hemoglobin tetramers, releasing each globin chains.
Solution A: 5% ACN, 0.1% TFA in deionized-water
Solution B: 95% ACN, 0.1% TFA in deionized-water
METHODS
Cleaning procedures
Before each run, LC column is rinsed using the following procedure:
Put in 2 solvent reservoirs 100% deionized-water and 100% ACN, respectively.
Rinse the column using a slow gradient (1-2%/min) from 5% to 95% ACN and back to 5% ACN.
Put in 2 solvent reservoirs solution A and solution B, respectively.
Acquisition procedures
A flow rate of 1.0 mL/min is applied with a gradient of solution A and solution B (increasing over time the hydrophobicity) following the parameters reported in Table 1.
First 2 blanks are run: mobile phases are injected into the LC column establishing the chromatogram background.
The diluted aliquot of Lyphochek Hemoglobin A2 control and then samples are injected (35 µl) into the mobile phase.
The absorbance is measured at 220 nm and each globin chain is identified based on the different retention time (lower -> hydrophilic; higher -> hydrophobic; Figure 1).
Quantification of each globin chain is performed using the Area under the Curve (AUC) (Figure 1) with the LC Solution Software.
| Time (min) | % RP-HPLC Buffer A | % RP-HPLC Buffer B |
10 | 60.8 | 39.2 |
27 | 59.9 | 40.1 |
34 | 59 | 41 |
42 | 58.5 | 41.5 |
51 | 58.1 | 41.9 |
55 | 57.2 | 42.8 |
59 | 57 | 43 |
65 | 56.8 | 43.2 |
67 | 55.4 | 44.6 |
69 | 55.4 | 44.6 |
74 | 0 | 100 |
79 | 0 | 100 |
82 | 60.8 | 39.2 |
95 | STOP | STOP |
Table 1: RP-HPLC gradient scheme for globin chains analysis. The gradient is designed with a high percentage of solvent A at the beginning and ends with 100% of solution B percentage (74-82 min) at a flow rate of 1.0 mL/min. The acquisition lasts 95 min per sample. Solution A is the limiting solution: 47.5 mL is used per sample.
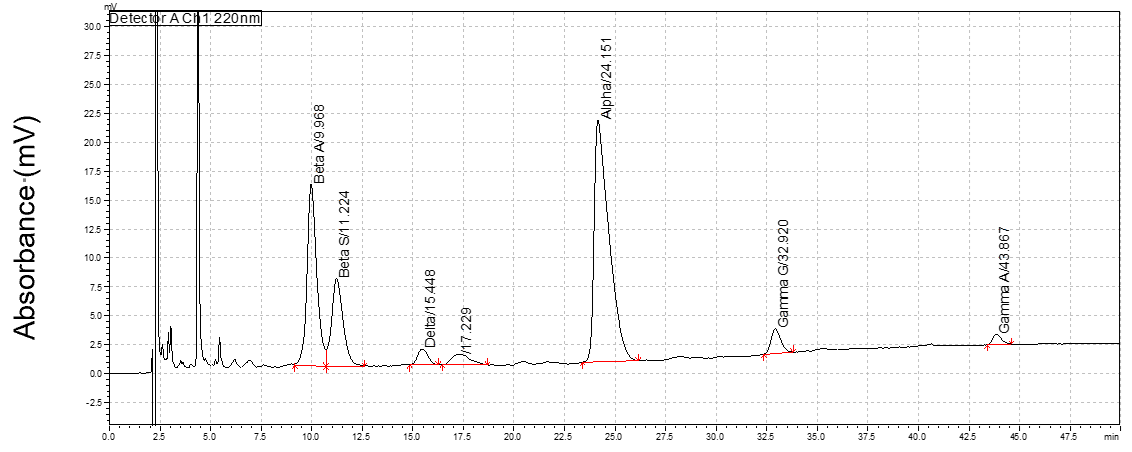
Peak# | Name | Retention Time (min) | Area | Height | Area% |
1 | Beta A | 9.968 | 527205 | 15627 | 12.005 |
2 | Beta S | 11.224 | 298779 | 7575 | 6.804 |
3 | Delta | 15.448 | 48067 | 1366 | 1.095 |
5 | Alpha | 24.151 | 1061331 | 20797 | 24.168 |
6 | Gamma G | 32.92 | 72452 | 2131 | 1.65 |
7 | Gamma A | 43.867 | 26743 | 877 | 0.609 |
Figure 1: RP-HPLC elution profile from Lyphochek Hemoglobin A2 control (Biorad) carrying different types of globin chains, eluted in the following order from the hydrophilic to the hydrophobic globins: ßA, ßS, δ, α, Aγ, Gγ. The peak at the elution time of 4.4 is corresponding to the heme from the hemoglobin.
- Chalumeau, A, Frati, G and Miccio, A(2020). Reverse phase(RP)-high-performance liquid chromatography (HPLC) analysis of globin chains. Bio-protocol Preprint. bio-protocol.org/prep420.
- Weber, L., Frati, G., Felix, T., Hardouin, G., Casini, A., Wollenschlaeger, C., Meneghini, V., Masson, C., Cian, A. D., Chalumeau, A., Mavilio, F., Amendola, M., Andre-Schmutz, I., Cereseto, A., Nemer, W. E., Concordet, J., Giovannangeli, C., Cavazzana, M. and Miccio, A.(2020). Editing a γ-globin repressor binding site restores fetal hemoglobin synthesis and corrects the sickle cell disease phenotype. Science Advances 6(7). DOI: 10.1126/sciadv.aay9392
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link


