Advanced Search
Overexpression and purification of the recombinant β-actin inclusion-body protein
Last updated date: Jul 21, 2020 Views: 1097 Forks: 0
β-actin production in E. coli
Scrape some of the frozen bacteria, harboring pCOLD I plasmid encoding the full-length human β-actin, off of the top of a glycerol stock and streak them onto an LB agar plate containing ampicillin (100 µg/ml). Grow the culture overnight at 37°C.
The construct can be requested from its creator – dr. Minoru Tamura (Ehime University, Japan) or if not available, from dr. Jakub Drozak (jdrozak@biol.uw.edu.pl).Pick a single colony from the LB agar plate and inoculate 55 ml of LB (100 µg/ml ampicillin) to start the preculture. Grow it on shaker (180 rpm) overnight at 30°C (E.coli BL21 DE3). The next day transfer 50 ml of the preculture to 500 ml LB with the antibiotic and grow at 37°C until its OD600 ≈ 0.5 A.U. To start the recombinant protein production, add IPTG to the final concentration of 200 µM and place the culture flask(s) on ice for 30 min (cold shock). Grow the culture for next 20 h at 15°C.
Harvest cells by centrifugation (6000 × g, 10 min) and store the bacteria pellet at -20°C until it will be used in a further procedure.
Cell lysis and solubilization of inclusion body β-actin
Resuspend the cell pellet in 27.5 ml of ice-cold lysis buffer by vortexing and transfer the resuspension to a 50 ml Falcon tube. Next, add 1.5 ml egg-white lysozyme (4 mg/ml H2O) and 250 µl Viscolase (1000 U in 1M MgSO4), and vortex briefly. Keep the resuspension on ice.
- Lyse the cells by tree cycles of freezing in liquid nitrogen and thawing at room temperature and incubate the lysates on ice for further 30 min.
- Pellet the insoluble proteins by centrifugation (20 000 × g, 20 min) and carefully decant the supernatant liquid.
- Completely dissolve protein pellet in 25 ml of wash solution A. Use Potter-Elvehjem homogenizer with glass pestle to solubilize the pellet thoroughly. Perform this procedure at room temperature.
- Pellet the insoluble proteins by centrifugation (20 000 × g, 10 min) and carefully decant the supernatant liquid.
- Repeat steps 7 and 8 with the use of wash solution B instead of wash solution A.
- Repeat steps 7 and 8 with the use of wash solution C instead of wash solution A.
- Completely solubilize the pellet of insoluble proteins in 30 ml of protein solubilization buffer by vortexing. The protein solution can be stored at a fridge for a few days.
Ni2+-affinity chromatography
The subsequent procedures should be carried out employing a FPLC system at ≈6°C.
- Prepare loading buffer (= protein solubilization buffer), refolding buffer and elution buffer and connect them to the FPLC.
- Centrifuge the protein solution resulting from step 11 (20 000 × g, 20 min) and collect the supernatant liquid (≈30 ml).
- Wash HisTrap FF 5 ml column with 20 ml of deionized H2O and equilibrate in 20 ml of loading buffer.
- Load 30 ml of the protein solution (the preparation from step 13) onto the column at 1.5 ml/min.
- Wash the column with 20 ml of loading buffer (1.5 ml/min) to remove weakly bound proteins.
- Perform β-actin refolding by washing the column with a 30 ml linear gradient, running from 0 to 100% refolding buffer (and from 100 to 0% loading buffer ) at 1.5 ml/min.
- Wash the column with 100% refolding buffer for 10 min at 1.5 ml/min.
- Elute the bound proteins by sequential washing the column with the following mobile phases:
a) 6% elution buffer and 94% refolding buffer, 1.5 ml/min, collect 20 ml (40 mM imidazole fraction).
b) 10% elution buffer and 90% refolding buffer, 1.5 ml/min, collect 20 ml (60 mM imidazole fraction).
c) 100% elution buffer, 1.5 ml/min, collect 20 ml (500 mM imidazole fraction).
Dialysis
As the refolded recombinant β-actin is eluted in “500 mM imidazole fraction”, immediately transfer this eluate to the dialysis bag (MWCO 14,000 Da) and start the dialysis (do not store the eluate, since purified recombinant β- actin tends to form an insoluble precipitate at high imidazole concentration).
Dialyze the fraction twice against 200 ml of dialysis buffer A (2 x 2h), then transfer the bag to 200 ml of dialysis buffer B and dialyze the preparation overnight. The next day perform the final dialysis against 200 ml of dialysis buffer B for 2h.
- Aliquot the purified β-actin and store at -70°C.
The typical yield of purification is about 10 mg of homogenous recombinant β-actin from 500 ml E. coli culture.
Solutions:
- Lysis buffer
20 mM Hepes (pH 7.5), 1 mM DTT, 1 mM ADP (sodium salt), 0.5 mM PMSF, 2 µg/ml leupeptin, 2 µg/ml antipain (prepare 30 ml). - Wash solution A
20 mM Hepes (pH 7.5), 2 M urea, 0.5 M NaCl, 5 mM DTT, 2 mM EDTA (prepare 25 ml). - Wash solution B
20 mM Hepes (pH 7.5), 0.5 M NaCl, 5 mM DTT, 2 mM EDTA (prepare 25 ml). - Wash solution C
20 mM Hepes (pH 7.5), 0.5 M NaCl (prepare 25 ml). - Protein solubilization buffer / loading buffer
20 mM Tris-HCl (pH 7.5), 6 M guanidine HCl, 0.5 M NaCl, 10 mM imidazole (prepare 300 ml) - Refolding buffer
20 mM Tris-HCl (pH 7.5), 0.5 M NaCl, 10 mM imidazole (prepare 300 ml) - Elution buffer
20 mM Tris-HCl (pH 7.5), 0.5 M NaCl, 500 mM imidazole (prepare 300 ml) - Dialysis buffer A
20 mM Tris-HCl (pH 7.5), 0.5 M NaCl, 1mM DTT (prepare 400 ml) Dialysis buffer B
20 mM Tris-HCl (pH 7.5), 1mM DTT, 6% sucrose (w/v) (prepare 400 ml)
Note that the pH adjustment should be performed on a buffer solution that contains all its ingredients.
Specific materials or reagents:
- Egg-white lysozyme (LYS702, Bioshop, Canada)
- Viscolase (1010-100, A&A Biotechnology, Poland)
- Dialysis tubing cellulose membrane (D9777, Sigma-Aldrich, USA)
- HisTrap Fast Flow 5 ml column (17-5255-01, GE Healthcare, USA)
Equipment
- Incubator and shaking incubator at 37 °C
- Floor-standing centrifuge
- Potter-Elvehjem homogenizer with glass pestle
FPLC
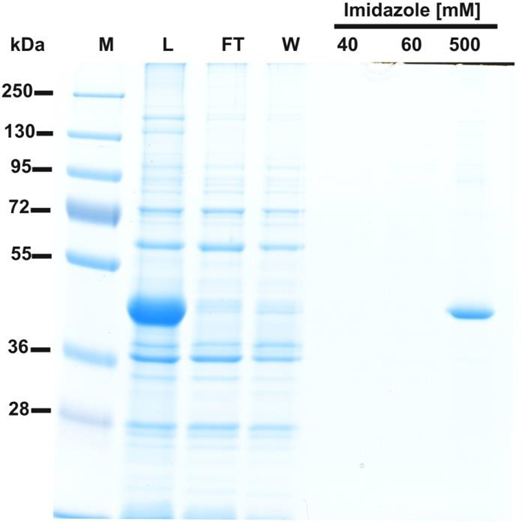
SDS-PAGE analysis of fractions obtained during purification of recombinant human β-actin overexpressed in E. coli.
20 µl of sample from each fraction was mixed with Laemmli buffer (4x concentrated) and loaded onto a 10 % gel, electrophoresed and the resulting gel was then stained with colloidal Coomassie blue. M, prestained protein marker; L, urea-washed inclusion bodies of E. coli applied on the column; FT, flow through; W, wash; Fractions 40 to 500 were eluted with the indicated concentrations of imidazole. Note that fractions L, FT and W were dialyzed against a buffer containing 8M urea instead of guanidine chloride prior to their denaturation and loading on the gel.
- Drozak, J(2020). Overexpression and purification of the recombinant β-actin inclusion-body protein. Bio-protocol Preprint. bio-protocol.org/prep407.
- Guo, Q., Liao, S., Kwiatkowski, S., Tomaka, W., Yu, H., Wu, G., Tu, X., Min, J., Drozak, J. and Xu, C.(2019). Structural insights into SETD3-mediated histidine methylation on β-actin. eLife. DOI: 10.7554/eLife.43676
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
