Advanced Search
hiPSC maintenance and differentiation
Last updated date: May 22, 2020 Views: 1362 Forks: 0
Detailed Protocol for hiPSC maintenance and differentiation into cerebrocortical neurons
Swagata Ghatak, Dorit Trudler, Nima Dolatabadi, Stuart A. Lipton.
hiPSC maintenance-
- Coat 6-well tissue culture plates with Matrigel at 0.09 mg/ml for 24 hours at room temperature.
- Plate hiPSC at 500,000 cells/well in mTESR+.
- Replace media every day.
- Monitor hiPSC colonies using a dissection microscope, and manually pick colonies or colony edges that look differentiated.
- When cells reach 80-90% confluence, split them:
- Wash cells with PBS without Ca2+ and Mg2+ for 1 minute.
- Add 1ml of gentle cell dissociation reagent.
- Incubate at 37°C for 7 minutes, or until the edges start to lift (every cell type is different and may require different incubation period and is density dependent).
- Remove the dissociation buffer gently and add media
- Dislodge the cells in media and collect them
- Plate the cells at a 1:3 to 1:10 ratio in media and add ROCK inhibitor for the first day (2 μM Thiazovivin).
- To freeze cells:
- Wash cells with PBS without Ca2+ and Mg2+ for 1 minute.
- Add 1ml Accutase.
- Incubate at 37°C for 5 minutes.
- Collect the cells in accutase.
- Wash the wells with media and combine with the collected cells.
- Centrifuge at 300xg.
- Remove supernatant and add Bambanker (for 1 well of cells add 2 ml of Bambanker) (3-5x10^6 cells/vial).
- Freeze in cryovials.
Differentiation of hiPSCs into cerebrocortical neurons (~90-95% glutamatergic, 5- 10% GABAergic neurons)-
1. Neuronal Induction:
- Upon reaching 80-90% confluence, dissociate hiPSCs under feeder free conditions with 1 ml accutase/ well.
- hiPSCs are placed in an aggrewell plate at a density of 3x10^6 cells per well of the aggrewell plate in *hESC medium supplemented with 2μM Dorsomorphin, A83-01 and PNU-74654 (DAP) for 5-7 days with 2 ml hESC medium/ well. During the sphere induction, include 10 µM Rock inhibitor Thiazovivin along with the other small molecule cocktail.
Alternatively, seed the hiPSCs at a density of 3-4x10^6 cells per well in a 6 well plate in 3 ml hESC medium supplemented with 2 μM Dorsomorphin, A83-01 and PNU-74654 (DAP) for 5-7 days (4-6 feeds). On the first day, include 10 µM Rock inhibitor Thiazovivin.
2. Neurosphere Phase:
a. After neural induction with DAP molecules (5-7 days), remove embryoid bodies (EBs) from the well by forcefully putting 1 ml of hESC medium (+DAP).
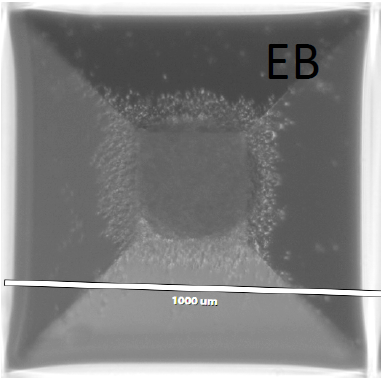
Alternatively, for the monolayer induction, manually make a grid of cells and scrape the cells as small squares that will form EBs.
b. Collect all the EBs (which are Pax-6 positive) and plate in a 6 well plate coated with 0.18 mg/ml matrigel (2X). The day of replating include 2 μM thiazovivin in the medium.
c. Feed every day with 3 ml of *hESC medium + DAP for 1.5-2 weeks. You should be able to see rosettes forming after 2-3 days, and most of the plate should be covered with cells.
3. Neural progenitor Cell (NPC) Expansion Phase:
- After two weeks, of continued maintenance in hESC medium and DAP, dissociate the cells with 1 ml accutase/ well and plate on a 6-well plate coated with 2X Matrigel in Neural medium supplemented with 20 ng/ml bFGF and 2 mM thiazovivin at a density of 5x10^6/well (1:2 replate).
- Approximately 24 hours after replating, perform a full medium change with Neural Medium supplemented with 20 ng/ml bFGF and 2 μM thiazovivin.
- Change medium every 48 hr with Neural Medium with 20 ng/mL bFGF.
- Upon confluence (usually 6-7 days after replating) dissociate cells with accutase and replate at 5x10^6 cells per well in a 2x Matrigel coated dish.
- Repeat steps 3b-3d until the third passage.
- From passage 4 onwards, replate cells in poly-L-ornithine/laminin (2 µg/cm2 and 1 µg/cm2, respectively)-coated tissue culture plates.
For subsequent passages, dissociate cells with accutase (for first dissociation – about 5-10 min; following dissociations are 3-5 min each) and maintain on poly- L-ornithine/laminin-coated tissue culture plates in Neural medium supplemented with 20 ng/ml bFGF. Change medium every 2 days.
- The cells/NPCs should be maintained at high density. Plate them at 3- 5x10^6/well after every split.
Important! Make sure to freeze and bank cells starting from passage 2. Try to expand as much as you can and freeze until passage 5, and then start conducting experiments.
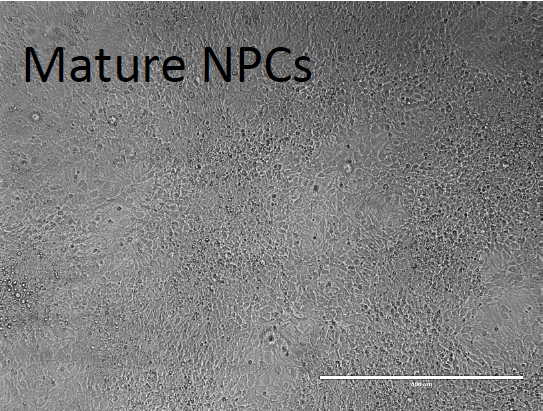
4. Terminal Differentiation without astrocytes:
Dissociate NPCs with 1 ml accutase/well and plate on 0.1% polyethyleneimine (PEI)/50 µg/ml poly-D-L-ornithine (PDLO)/4 µg/ml laminin coated coverslips (in 24-well plates) at a density of 350,000-400,000 NPCs/coverslip.
- Initially, maintain terminally differentiating cells in Neural medium supplemented with 0.1 µM Compound-E (gamma secretase inhibitor) for 2 days.
- After 2 days, switch terminally differentiating cells to Neural medium supplemented with 20 ng/ml each of hBDNF and hGDNF.
- Change media every 3-4 days or upon visual acidification as indicated by phenol red.
- After 1 week change medium to BrainPhys medium. supplemented with 20 ng/ml each of hBDNF and hGDNF.
- Alternatively, at step b, maintain terminally differentiating cells in 1 ml BrainPhys medium supplemented with 0.1 µM Compound-E for 2 days. Add fresh 0.5-1ml BrainPhys medium supplemented with 20 ng/ml each of hBDNF and hGDNF after 2 days. Subsequently do half media change every 3-4 days with the same medium.
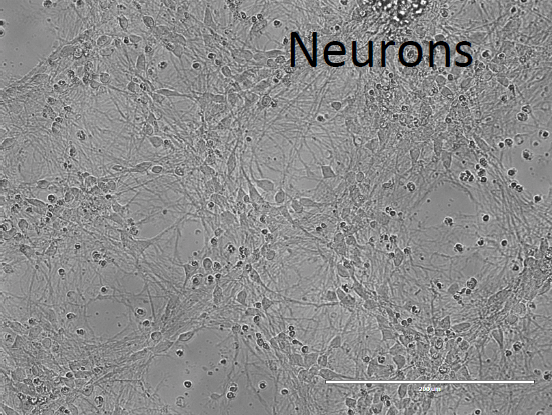
5. Terminal Differentiation with mouse astrocytes:
- Prepare astrocytes from postnatal day (P)2-3 mice [Liu et al., 2006].
- Dissociate astrocyte culture and plate on 24 well plate, on cover slips coated with poly- L-ornithine/laminin at a density of 50,000 cells/well.
3 days later dissociate NPCs and replate onto astrocytes at a density of 50,000 cells/well in Neural medium supplemented with 0.5% FBS and 20 ng/ml each of hBDNF and hGDNF.
- Change medium every 3-4 days (half-medium change).
- After 1 week, change medium to BrainPhys supplemented with 20 ng/ml each of hBDNF and hGDNF.
*Media compositions:
hESC medium- For 500ml
- DMEM-F12 + GlutaMAX = 500 ml
- Knockout serum replacement = 20%
- Non-essential amino acids = 1x
- β-Mercaptoethanol = 100 µM
Neural medium- For 500ml
- DMEM-F12 + GlutaMAX = 500 ml
- BSA Fraction V = 0.015%
- β-Mercaptoethanol = 100 µM
- N2 Supplement = 1x
- B27 Supplement = 1x
Reagents used in the protocol-
S.No. | Reagent | Source | Catalog number |
1 | Matrigel | Corning | 354230 |
2 | mTESR+ | STEMCELL Technologies | 05825 |
3 | Gentle cell dissociation reagent | STEMCELL Technologies | 07174 |
4 | Dorsomorphin | Tocris | 3093 |
5 | A83-01 | Stemgent | 04-0014 |
6 | PNU74654 | Tocris | 3534 |
7 | DMEM/F12 + Glutamax | ThermoFisher | 10565018 |
8 | N2 | ThermoFisher | 17502048 |
9 | B27 | ThermoFisher | 17504044 |
10 | FGF | R&D | 4114-TC |
11 | Poly L-ornithine | Millipore Sigma | 27378490 |
12 | laminin | Trevigen | 340001001 |
13 | Knockout Serum Replacement | ThermoFisher | 10828028 |
14 | β-Mercaptoethanol | ThermoFisher | 21985023 |
15 | hBDNF | Peprotech | AF-450-02 |
16 | hGDNF | Peprotech | AF-450-10 |
17 | ES-FBS | ThermoFisher | 16141079 |
18 | Polyethyleneimine (PEI) | Millipore Sigma | P3143L |
19 | Thiazovivin | STEMCELL Technologies | 72254 |
20 | Aggrewell | STEMCELL Technologies | 34415 |
21 | BrainPhys | STEMCELL Technologies | 05794 |
22 | Accutase | Invitrogen | 00-4555-56 |
23 | BSA Fraction V | ThermoFisher | 15260037 |
24 | Compound-E | Millipore Sigma | 530509 |
25 | Poly-D- L-ornithine (PDLO) | Millipore Sigma | P0671 |
REFERENCE
1. Liu, H.T., Tashmukhamedov, B.A., Inoue, H., Okada, Y. & Sabirov, R.Z. 2006. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia 54, 343-357. doi: 10.1002/glia.20400.
- Ghatak, S, Trudler, D, Dolatabadi, N and Lipton, S(2020). hiPSC maintenance and differentiation. Bio-protocol Preprint. bio-protocol.org/prep323.
- Ghatak, S., Dolatabadi, N., Trudler, D., Zhang, X., Wu, Y., Mohata, M., Ambasudhan, R., Talantova, M. and Lipton, S. A.(2019). Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs isogenic controls. eLife. DOI: 10.7554/eLife.50333
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
