Advanced Search
ESC-derived motor neurons
Last updated date: Nov 21, 2019 Views: 1292 Forks: 0
Grow and differentiate inducible mESC to motor neurons
Day 1
- Prepare 3 mL 0.1% gelatin coating in T25 flask (incubate 15-30 min at RT and aspirate before use)
- Thaw frozen cells in the water bath
- Use 4.5 mL feeder medium to resuspend the cells and centrifuge 4’ at 900 rpm (156 g for RCF)
(hold 4.5 ml feeder medium in pipette, take in the 0.5 mL thawed cells, pipette out in a 15 mL falcon tube with ~2 mL medium left in the pipette to wash the cryo-vial once; try to minimize the time when cells are thawed and exposed to freezing medium) - Aspirate and add 5 ml of 80-20 medium (80% 2i medium & 20% Mouse ESC medium), pipette 3-4 times to resuspend, and plate the cells in T25
Day 2
Change medium (5 mL of 80-20 medium)
Day 3
(Day 3 or when the colonies grow to proper size but not merged; a good mESC colony should have a smooth and bright edge and the cells in it are not in a monolayer but in a 3-D colony)
- One wash with PBS (5ml)
- Add 1mL of TrypLE (cannot use trypsin with 2i medium, don’t need to warm the TrypLE before use) and wait ~1 min at RT (when you tilt or slightly shake the flask, you should see colonies detach and float)
- Add 4 mL feeder medium to block TrypLE and pipette 3-4 times to dissociate the colonies (with tip of pipette against the corner of flask to enhance the power), transfer to a 15 mL tube and centrifuge 4 min at 900 rpm
- Aspirate and add 5-10 mL of AK medium to resuspend the cells
(if the cells are not single/not dissociated well, you can pipette several times more but might impair the cells so it’s kind of tricky; I usually first use 1 mL AK medium and the p1000 pipette to gently resuspend the cells by pipetting several times, then add medium to mix well) - Count and plate 300,000 cells in 12 mL AK medium per 100mm untreated dishes for suspension culture (to generate a large number of cells, see 3-4 million cells in 70 mL AK medium per 245mmX245mm big square plate)
Day 5
- Collect EBs in falcon tubes and centrifuge for 3 min at 700 rpm (94 g for RCF)
*If there are big or fused EBs, you can remove them individually or let the tube sit there for a moment and remove the big ones precipitated by gravity;
*For p245 plate, if there are a lot of EBs left on the bottom after collection, wash with 10 mL PBS to get all EBs). - Gently resuspend and plate the EBs in 12 mL AK medium per 100 mm plate. Doxycycline (Dox) (3µg/ml) should be added fresh.
*better have a control without Dox in the first time to test if the differentiation works
Day 7 (48h of Dox treatment)
Collect the EBs for cryosection (for immunostaining) or dissociate the EBs to single cells and plate (for immunostaining: plate the cells on coated coverslips).
*Some EBs may have sent processes and attached to the plate, make sure to collect all of them especially for p245 plates (the way I collect for p245 plate is to collect ~50 mL in the first tube, then use the left medium in the plate to try to wash off the EBs and collect them in the second tube, later use the supernatant medium in the first tube to wash off the left EBs attached, it can take several washes to get all the EBs).
Examples:
ESC colonies on Day 3 (10X)
Example of good ESC colonies (the colonies grow to proper size but not merged; a good mESC colony should have a smooth and bright edge and the cells in it are not in a monolayer but in a 3-D colony):
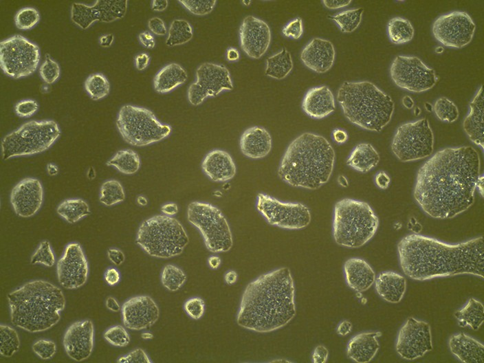
Example of good ESC colonies that over-grow:
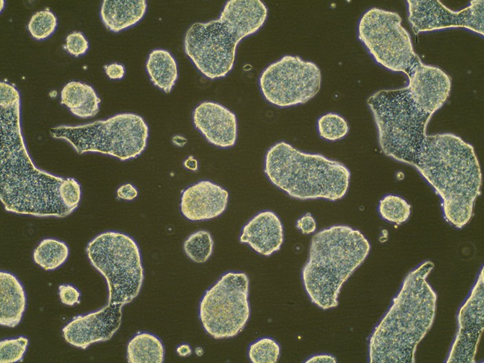
Example of bad ESC colonies:
Example of bad ESC colonies that over-grow:
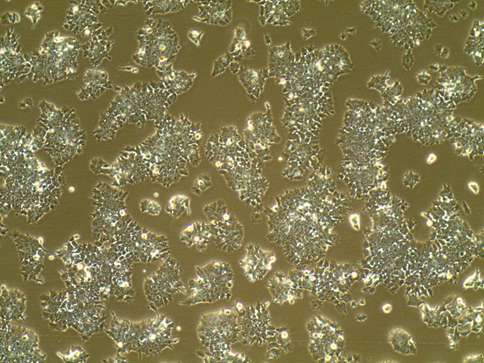
EBs on Day 5 (right before Dox addition)
Example of good EBs (10X):

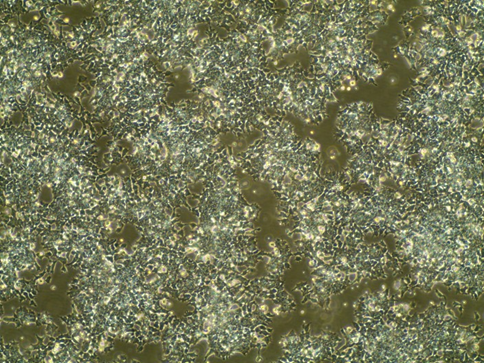
EBs on Day 7 (right before dissociation) (10X)
Example of EBs treated with Dox for 2 days (round, smooth surface, bright or yellowish; the huge ones may get a little black in the center):
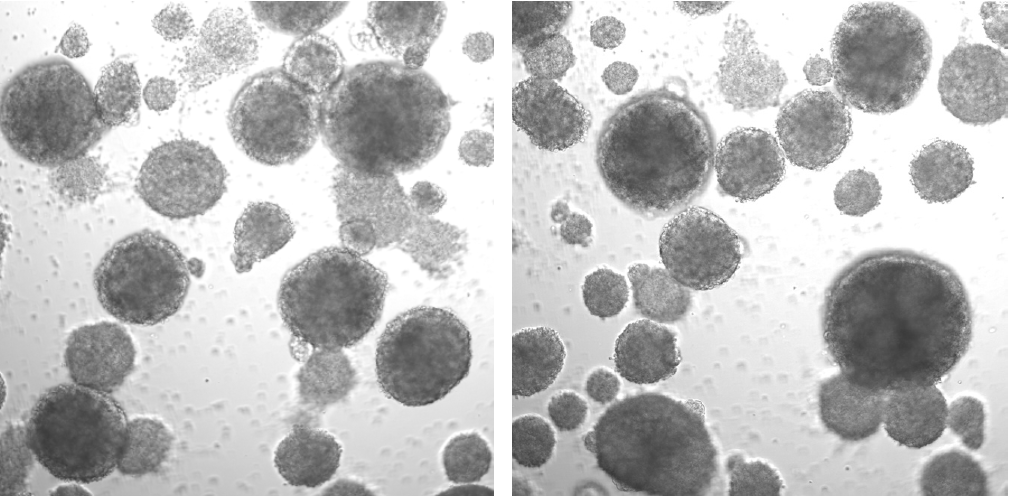
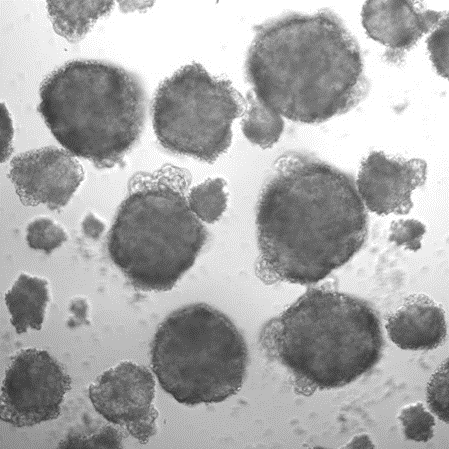
Example of EBs without Dox treatment for 2 days (irregular shape and more dead cells):
Expand cell lines (freeze cells)
- Two hours before starting the experiment: change medium for ESCs; prepare freezing medium, cryo vials and freezing box, and keep them in 4 degree.
*Label the cryo-vials with
Cell line/name Passage number Selection/marker
Format of flask or plate prepared for (e.g. T25) Date and initials
Other necessary information - When the ESCs reach a nice confluence (~70% confluence with big colonies but not merged, usually takes 2- 3 days), dissociate the cells with TrypLE, collect all cells (usually there are many cells at the edge of flask), count the number of cells (usually one T25 can produce from 4 to 7 millions), spin down 900 rpm 4 min.
- Resuspend the cells with proper amount of freezing medium, split into cryo vials, place in the freezing box and keep in -80 degree. (Usually use 0.5 mL to freeze 1~ 2 million cells per vial. When close the cryo vial, screw tight but not too tight to squeeze the ring between the cap and tube. This step should be done as fast as possible.)
- Transfer the aliquots to liquid nitrogen tank the following day for long term storage.
Mediums
| Feeders Medium | Vf ≈ 600 ml | [ ]f | Working | Stock | Cat # |
| Knockout™ DMEM | 500 ml | - | + 4°C, TC | + 4°C Freezer | Thermo Fisher, A1286101 |
| FBS | 50 ml | 10% | + 4°C, TC | -20°C Freezer (defrost o/n at +4°C) | VWR, 35-075-CV |
| L-Glutamine (100X) | 5 ml | 1X | + 4°C, TC | -20°C Freezer | Thermo Fisher, 25030081 |
| Pen/Str (100X)(when needed) | 5 ml | 1X | + 4°C, TC | -20°C Freezer | Thermo Fisher, 15070063 |
Filter and aliquot in 50ml Falcon tubes. Date and label the tubes.
| 3X Mix | Vf ≈ 150 ml | [ ]f | Working | Stock | Cat # |
| L- Glutamine | 50 ml | 1X | + 4°C, TC | -20°C Freezer | Thermo Fisher, 25030081 |
| NEAA (100X) | 50 ml | 1X | + 4°C, TC | -20°C Freezer | Fisher, NC9520478 |
| Nucleosides (100X) | 50 ml (1 bottle) | 1X | + 4°C, TC | -20°C Freezer | Fisher, NC9559754 |
| mESC Medium | Vf ≈ 300 ml | [ ]f | Working | Stock | Cat # |
| Knockout™ DMEM | 250 ml | - | + 4°C, TC | + 4°C Freezer | Thermo Fisher, A1286101 |
| FBS | 35 ml | 14% | + 4°C, TC | -20°C Freezer (defrost o/n at +4⁰C) | VWR, 35-075-CV |
| BME (100X) | 2.5 ml | 1X | + 4°C, TC | + 4°C Freezer | Thermo Fisher, 21985-023 |
| 3X Mix | 7.5 ml |
| + 4°C, TC | -20°C Freezer | see recipe, make ourselves |
| Pen/Str (100X)(when needed) | 2.5 ml | 1X | + 4°C, TC | -20°C Freezer | Thermo Fisher, 15070063 |
| LIF (107 U/ml) | 25 µl | 103 U/ml | + 4°C, TC |
| Fisher, ESG1107 |
Filter and aliquot in 50ml Falcon tubes. Date and label the tubes.
| 2i for mESC | Vf ≈ 420ml | [ ]f | Working | Stock | Cat # |
| Advanced DMEM/F12 | 200 ml | - | + 4°C, TC | + 4°C Freezer | Thermo Fisher, 12634028 |
| Neurobasal | 200 ml | - | + 4°C, TC | + 4°C Freezer | Thermo Fisher, 10888022 |
N2 (100X) |
4 ml |
1X | -20°C Freezer, Box N2/B27 |
|
Thermo Fisher, 17502-048 |
B27 (50X) |
8 ml |
1X | -20°C Freezer, Box N2/B27 |
|
Thermo Fisher, 17504-044 |
| L-Glutamine (100X) | 4 ml | 1X (2mM) | + 4°C, TC | -20°C Freezer | Thermo Fisher, 25030081 |
| BME (100X) | 4 ml | 1X (0.1mM) | + 4°C, TC | + 4°C Freezer | Thermo Fisher, 21985-023 |
| LIF (107 U/ml) | 50 µl | 103 U/ml | + 4°C, TC |
| Fisher, ESG1107 |
| CHIR99021 (3 mM) | 400 µl | 3 µM | + 4°C, TC | -20°C Freezer, Box2 Inhibitors | Biovision, 1991 |
| MEKi / PD0325901 (1 mM) | 400 µl | 1 µM | + 4°C, TC | -20°C Freezer, Box1 Inhibitors | Sigma, PZ0162-5MG |
| Pen/Str (100X)(when needed) | 4 ml | 1X | + 4°C, TC | -20°C Freezer | Thermo Fisher, 15070063 |
Filter and make 40ml aliquots. Add 10 ml of mESC medium to have final 80%2i/20% mESC medium |
| ||||
| mDifferentiation Medium (AK) | Vf ≈ 590ml | [ ]f | Working | Stock | Cat # |
| Advanced DMEM/F12 | 250 ml | - | + 4°C, TC | + 4°C Freezer | Thermo Fisher, 12634028 |
| Neurobasal | 250 ml | - | + 4°C, TC | + 4°C Freezer | Thermo Fisher, 10888022 |
| Knockout-SR | 75 ml | 10% | + 4°C, TC | -20°C Freezer (defrost o/n at +4°C) | Thermo Fisher, 10828-028 |
| BME (100x, but we use less) | 2.5 ml | 1 X | + 4°C, TC | + 4°C Freezer | Thermo Fisher, 21985-023 |
| L-Glutamine (100X) | 5 ml | 1X | + 4°C, TC | -20°C Freezer | Thermo Fisher, 25030081 |
| Pen/Str (when needed) | 5 ml | 1X | + 4°C, TC | -20°C Freezer | Thermo Fisher, 15070063 |
Filter and aliquot in 50ml Falcon tubes. Date and label the tubes.
| Freezing Medium |
|
| [ ]f | Cat # |
| ES-FBS (the same you use to grow the cells) |
|
|
50% |
VWR, 35-075-CV |
Growth medium (the same you use to grow the cells) |
|
|
40% |
80%2i/20%ES, make ourselves |
| DMSO (sterile from TC room) |
|
| 10% | Sigma, D2650-100ML |
FBS Stock inactivation: 30 min @ 55°C (previously thawed o/n @ +4°C)
Dissociate EBs to single neurons
1. Collect EBs in a 15 mL tube and spin down at 700 rpm (94 g for RCF) 3 min (also collect the EBs attached to the bottom of plate)
2. Wash with 10 mL PBS and spin down at 700 rpm, 3 min
3. Add 1 mL 0.05% Trypsin-EDTA to resuspend the EBs, keep in room temperature for 1-3 min (the EBs will stick to each other and you will see a white “thread” when flip the bottom of the tube)
4. Add 4 mL feeder medium to block the trypsinization and pipette 7~13 times (usually 10 times) with the pipette tip against the bottom corner of the tube, try to avoid foaming and stop pipetting when there is no visible EBs (if there is still some precipitate, leave it to the next step, do not pipette too much). Spin down at 900 rpm (156 g for RCF) 4 min
5. Resuspend the cells with 1 mL AK medium by p1000 pipette.
i. Check the cells with hemocytometer (If there is precipitate left from the previous step, pipette 5~10 times with p1000 tip before checking).
*If you see some white thread-like precipitate by eye, just take it out by tip.
ii. Under microscope, if there are EBs or chunks of cells while single cells are required, pipette more with p1000 tip and check again until most of them are single cells.
*However, do not keep pipetting if the cells become dying (e.g. most cells are grey or with crinkled surface). The undissociated chunks could be filtered out or precipitate to the bottom of the tube by gravity.
iii. Add another 4 mL AK medium to wash the cells and spin down at 900 rpm, 4 min. (If there are a lot of dead cells and debris, wash twice or at most three times with AK medium)
6. Resuspend the cells with 1~3 mL motor neuron medium, count with hemocytometer and plate.
*The protocol above is applicable to 1-2 plates of EBs. If you have more EBs, use a 50 mL Falcon tube, a proper amount of trypsin (2~4 mL) and adjust the amount of other mediums used. For example, if generate EBs in the 245mmX245mm big square plate, I usually split the EBs into two 50 mL Falcon tubes to trypsinize separately using 3 mL trypsin, incubate RT 2~4 min according to the number of EBs and the dissociation difficulty of the cell line, and then block with maximum 9 mL feeder medium so that I can use a 10 mL serological pipette to well dissociate the EBs (try to take nearly everything during each pipette-in and -out, and make sure the pipette tip is against the corner of the tube not the flat bottom of the tube). Merge dissociated cells from the two 50 mL tubes into a 15 mL tube as soon as possible (usually after spinning down), because aspiration from the pellet in 50 mL tube could cause cell loss.
*A nice density to count with hemocytometer is 1~2 million per mL.
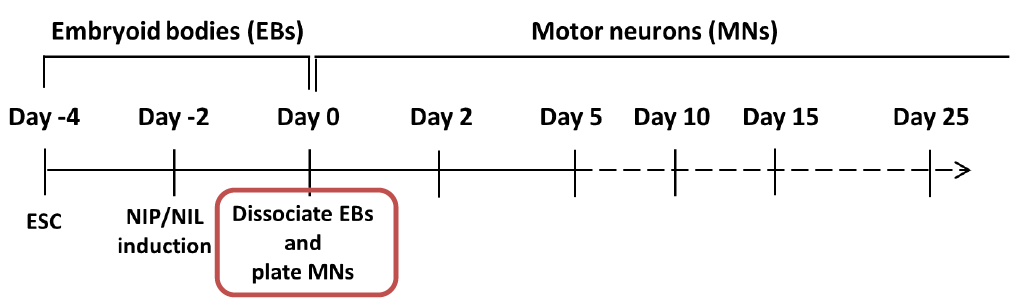
Schematic protocol of motor neuron differentiation and maintenance
(The step in the red box is described as above)
Culture conditions of motor neurons
i) Coating
- Poly-L-Ornithine or Poly-D-Lysine alone is enough for neurons to form nice networks. There is no observed difference between these two coatings.
Substrate | Final concentration | Polymerization | Wash (twice) | To Dry? | Cat # |
Poly-L- Ornithine | 0.001% (use 1:10 from 0.01% stock) | 2-48 h, 37 ℃ incubator | H2O | Y | Sigma |
Poly-D- Lysine | 0.001% (use 1:10 from 0.01% stock) | 2-48 h, 37 ℃ incubator | H2O | Y | Sigma P0899-10MG |
Matrigel | 1X (use 1:100 from 100X stock diluted in Neurobasal) | 2-48 h, 37 ℃ incubator | H2O | N | BD Bioscience 356231 |
Laminin | 5 ug/uL (use 1:100 from stock) | 2-3 h, 37 ℃ incubator | H2O | N | Fisher Scientific CB-40232 |
Plates | Volume per well for coating |
96-well plate | 30-50 uL |
24-well or 4-well plate | 250 uL (200 uL minimum) |
24-well or 4-well plate with round coverslips | Only on the coverslip: ~110 uL; Whole well: 250 uL minimum |
60mm plate | 1.5 mL |
100mm plate | 5 mL |
150mm plate | 10 mL |
*Laminin is usually used to coat over poly-L-Ornithine or poly-D-Lysine, which will improve cell movement and axon growth. While we haven’t seen significant improvement in survival with the laminin we have (even at 10 ug/uL), using the commercial laminin/poly-D-lysine 8-well chambers does have a effect.
*Matrigel will improve survival but also tend to preserve the undifferentiated cells even with FDU/Uridine present.
*To coat 8-well chambers with poly-D-lysine, higher concentration is required. 0.01% poly-D-lysine (10X) has been tested (as recommended in the sigma website, incubate 5 min at RT, wash twice and dry for 2 hours). It works but may be too sticky for neurons.
ii) Supplement
Substrate | Concentration | Function | Cat # |
Doxycycline | 3 ug/mL | Induction of transgenic genes | Sigma D9891-1G |
Retinoic acid | 0.5-1uM | Induce cervical fate if needed | Sigma R2625-50MG |
FDU & Uridine | 4 uM of each | Toxic to proliferating cells | Sigma F0503-100MG; U3003-5G |
GDNF | 10 ng/mL | Alone is sufficient to prolong survival. | PeproTech 450-10 |
Forskolin | 10 uM | Prolong survival. Toxic for glia cells | Fisher BP2520-5 |
IBMX | 100 uM | Prolong survival. | Tocris 2845 |
BDNF | 10 ng/mL | Prolong survival | PeproTech 450-02 |
CNTF | 10 ng/mL | Prolong survival | PeproTech 450-13 |
*Without any neurotrophic supplement, neurons cannot survive to the 4th day.
*Currently, I’m using this supplement cocktail: 3 ug/mL Dox + 4 uM FDU & 4 uM Uridine + 10 ng/mL GDNF + 10 ng/mL BDNF + 10 ng/mL CNTF + 100 uM IBMX + 10 uM Forskolin
P.S. “Dox + FDU & Uridine + 1 ng/mL GDNF + 100 uM IBMX + 10 uM Forskolin” was used previously and okay for short-term culture less than a week, but with much lower survival rate.
*Supplements should be added fresh (no more than one week if kept in 4 degree).
iii) Density
For 96-well (0.3 cm2 per well): 5000-10000 cells seem nice within 150-200 uL medium per well.
For 4-well or 24-well plates (2 cm2 per well): To have absolute single neurons, use 5000 cells per well. For regular purposes, plate ~30,000 cells per well within 0.5 mL medium.
For p60 plate (20 cm2): 500,000 cells maximum, within 3 mL medium.
For p100 plate (60 cm2): 1 million cells for long-term culture, within minimum 6 mL medium (if plated a lot more than 1 million, there will be much more cell death since day 3~4 especially for NIP-induced cranial motor neurons).
For p150 plate (140 cm2): I’ve tried 8 million cells within 12 mL, fine for 2 days at least.
Motor Neuron Medium (MNM)
Motor Neuron Medium | Vf ≈ 100ml | [ ]f | Working | Stock |
Neurobasal | 100 ml | - | + 4°C, TC | + 4°C Freezer |
FBS | 2 ml | 2% | + 4°C, TC | -20°C Freezer (defrost o/n at +4°C) |
B27 (50X) | 2 ml | 1X | -20°C Freezer, Box N2/B27 | |
L-Glutamine (100X) | 250 ul | 0.5 mM | + 4°C, TC | -20°C Freezer |
BME (100X) | 100 uL | 0.01mM | + 4°C, TC | + 4°C Freezer |
Neurobasal [Invitrogen 10888-022]
FBS [Corning 35-075-CV]
B27 (50X) [Invitrogen 17504-044]
L-Glutamine [Invitrogen 25030-081]
2-mercaptoethanol [Invitrogen 21985-023]
(1 mL Pen/strep 100X if needed [Invitrogen 15070-063])
*The 100 uL Glutamate(E) in the original protocol hasn’t been used.
*10% Horse serum may be better than 2% FBS, more tests needed
- Mazzoni, E O(2019). ESC-derived motor neurons. Bio-protocol Preprint. bio-protocol.org/prep29.
- An, D., Fujiki, R., Iannitelli, D. E., Smerdon, J. W., Maity, S., Rose, M. F., Gelber, A., Wanaselja, E. K., Yagudayeva, I., Lee, J. Y., Vogel, C., Wichterle, H., Engle, E. C. and Mazzoni, E. O.(2019). Stem cell-derived cranial and spinal motor neurons reveal proteostatic differences between ALS resistant and sensitive motor neurons. eLife. DOI: 10.7554/eLife.44423
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
