Advanced Search
Immunostaining protocol of human 3D bioengineered skeletal muscle whole mounts and cross section for confocal microscopy
Last updated date: Mar 26, 2024 Views: 889 Forks: 0
Immunostaining protocol of human 3D bioengineered skeletal muscle whole mounts and cross section for confocal microscopy
Arne D. Hofemeier, Timo Betz
Third Institute of Physics – Biophysics, University of Göttingen, Germany
Graphical Abstract
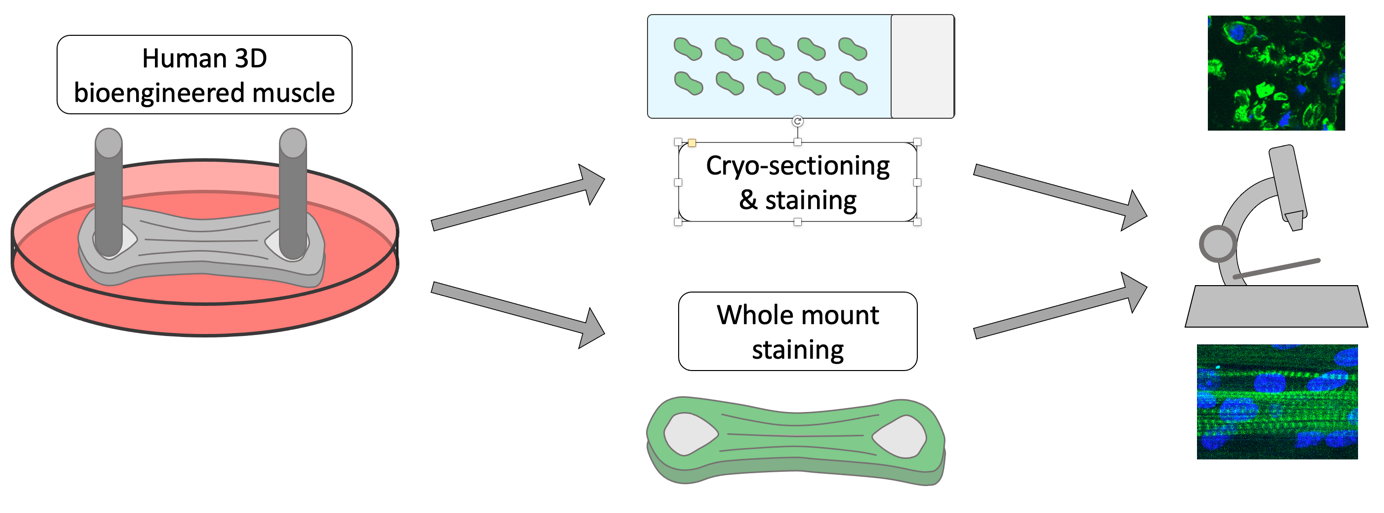
This protocol describes fixation, freezing, cryo-sectioning and immunostaining of human 3D bioengineered skeletal muscle tissue in detail.
Fixation of human 3D bioengineered skeletal muscle
- Wash the tissue inside the tissue culture mold once with phosphate buffered saline (PBS).
- Incubate the tissue for 15 min at room temperature in 4% (w/v) paraformaldehyde (PFA) solution.
- Wash the tissue three times with PBS.
- Fixed tissue can now be removed from the culture mold carefully without structural changes using tweezers and stored in PBS at 4 °C.
Embedding and freezing of human 3D bioengineered skeletal muscle for cryo-sectioning
- Incubate fixed tissue in 30 % (w/v) sucrose solution for 48 h at 4 °C to dehydrate tissue.
- Fill a cryomold with OCT mounting media avoiding air bubbles.
- Take out the tissue from the sucrose solution and shortly touch a tissue paper carefully to remove remaining sucrose solution from the tissue, but do not dry it out.
- Transfer the tissue to the OCT mounting media and remember positioning of the tissue in the cryomold to assure cross-sectioning later (label cryomold).
- Freeze the tissue embedded in OCT using liquid nitrogen. To avoid cryodamage, freeze it slow by first holding the cryomold into the dust above the liquid nitrogen without actually touching the liquid nitrogen. The OCT mounting media will slowly turn white. Once the OCT mounting media is completely white/frozen, drop the cryomold into the liquid nitrogen. Store the samples at -80 °C until cryo-sectioning.
Cryo-sectioning of human 3D bioengineered skeletal muscle
- Prepare frozen cryo cross section at -21 to -24 °C.
- Adjust the temperature of the Cryostat machine and the samples to the sectioning temperature at least 1 h prior to start.
- Mount your sample for 90° cross sectioning and trim your sample until you reach the tissue
- Prepare 5 – 25 µm cross section (thickness may vary depending on purpose of the experiment) and transfer them on charged SuperFrost® microscope slides.
- Let them dry at room temperature and store them at -20 °C.
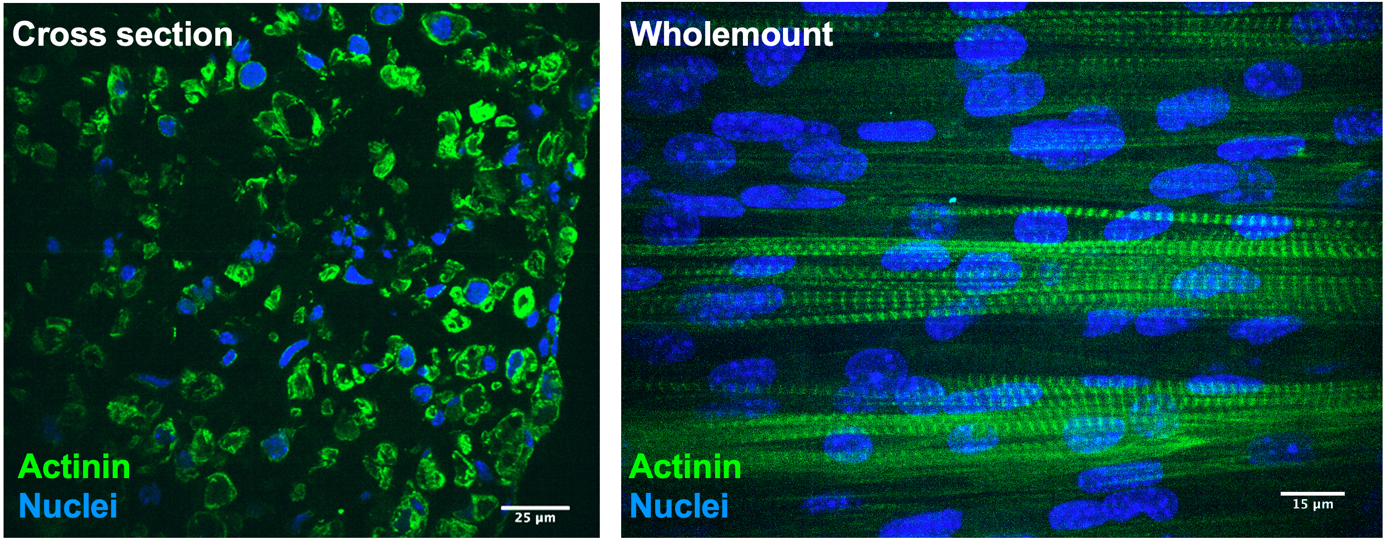
Immunostaining of human 3D bioengineered skeletal muscle cross section
- Rehydrate samples in PBS
- Incubate samples in blocking solution (PBS supplemented with 10% goat serum and 0.2% Triton-X-100) for 1h at room temperature
- Dilute the primary antibody to the manufacturer’s guidelines in blocking solution and incubate on the sample over night at 4 °C.
- Wash sample three times with PBS. For each washing let the PBS incubate with the sample for 10 min.
- Dilute the secondary antibody to the manufacturer’s guidelines in blocking solution and incubate on the sample for 45 min at room temperature.
- Wash sample three times with PBS. For each washing let the PBS incubate with the sample for 10 min.
- Mount sample in Fluoromount media underneath a coverslip and seal it with coverslip sealant.
- For best confocal imaging use 63x water immersion objectives with a numerical aperture above 1.1
- Store samples at 4 °C.
Immunostaining of human 3D bioengineered skeletal muscle whole mount
- Incubate fixed tissue in blocking solution (PBS supplemented with 10% goat serum and 0.3% Triton-X-100) for 1h at room temperature
- Dilute the primary antibody to the manufacturer’s guidelines in blocking solution and incubate on the sample over night at 4 °C.
- Wash sample three times with PBS. For each washing let the PBS incubate with the sample for 10 min.
- Dilute the secondary antibody to the manufacturer’s guidelines in blocking solution and incubate on the sample for 45 min at room temperature.
- Wash sample three times with PBS. For each washing let the PBS incubate with the sample for 10 min.
- For best confocal imaging we suggest 63x water immersion objectives with a numerical aperture above 1.1
- Store samples at 4 °C in PBS.
Materials
Cryostat (e.g Leica)
Paraformaldehyde (e.g. Sigma)
PBS (without calcium and magnesium) (e.g. Sigma)
Goat serum (e.g. Sigma)
Triton-X-100 (e.g. Sigma)
Antibodies for protein of interest
Sucrose (e.g. Sigma)
OCT mounting media (e.g CellPath)
Cryomolds (10 x 10 x 5 mm) (e.g. Tissue-Tek Cryomold®)
SuperFrost® charged microscope slides (e.g. Epredia)
Coverslips #1.5 (e.g. Marienfeld)
Fluoromount media (e.g. Invitrogen)
Coverslip sealant (e.g. Bioticum)
Tweezers (e.g. FST)
Liquid nitrogen
Images
- Hofemeier, A and Betz, T(2024). Immunostaining protocol of human 3D bioengineered skeletal muscle whole mounts and cross section for confocal microscopy. Bio-protocol Preprint. bio-protocol.org/prep2619.
- Hofemeier, A. D., Limon, T., Muenker, T. M., Wallmeyer, B., Jurado, A., Afshar, M. E., Ebrahimi, M., Tsukanov, R., Oleksiievets, N., Enderlein, J., Gilbert, P. M. and Betz, T.(2021). Global and local tension measurements in biomimetic skeletal muscle tissues reveals early mechanical homeostasis. eLife. DOI: 10.7554/eLife.60145
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
