Advanced Search
HSPC and MPP2 isolation, barcoding, EPO treatment, and transplantation
Last updated date: Feb 7, 2024 Views: 962 Forks: 0
Protocol for lentiviral barcoding of EPO treated hematopoietic stem and progenitor cells: Cell purification, barcoding, and transplantation
Study overview:
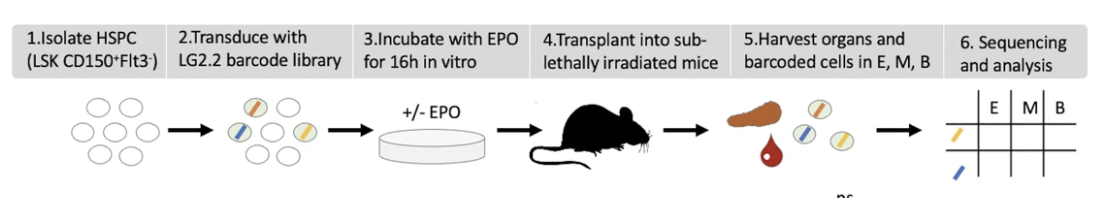
Figure 1: Overview of the experimental design (a) HSPCs were sorted from the bone marrow of donor mice, lentivirally barcoded, cultured ex vivo with or without 1000 ng/ml EPO for 16 hr, and transplanted into sublethally irradiated mice. At week 4 post-transplantation, the erythroid (E), myeloid (M), and B-cells
Material:
A full list of reagents and material used in this protocol is provided in the original article in the ‘Key resources’ table
Step 1- progenitor isolation
A. BM isolation: Bone collection and ckit enrichment
- Sacrifice mice
- 6 BL6N D45.1 Males aged 8 weeks
- Recover BM (tibia, femur, iliac)
- Take some cells for unstained and single stain controls
B. Flushing bones
- Put bones and medium into a 10 cm petri dish.
- Prepare the scissors and tweezer by rinsing with EtOH.
- Rinse before taking bone in petri dish medium to dilute EtOH.
- Prepare a 15 ml tube or 50 ml with 4-5 ml/ mouse cold PBS+ to collect the cells.
- Prepare a 2ml syringe with a long green needle (21G 50mm)
- fill it with 2ml cold PBS+ from the collection tube
- Put needle into tibia or non-star like end of femur.
- For iliac bone at V shaped end at the tip of the V.
- Take care to use pointiest part of needle.
- Put entire bone into cold PBS+ and eject 1ml of cold PBS+.
- For tibia and femur, turn the bone ¼ without detaching it, for iliac bone just put deeper into bone (should feel that is breaking a membrane), and eject other 1ml into the tube.
- If bone is not white yet, restart, cold PBS+ taken is from the collection tube!
- Empty bones can be collected in lid of petri dish.
- Between each bone, dissociate the red pulp by pipetting up and down with the syringe and ejecting against the tube wall.
- Never put tweezer into collection tube. If lose bone try to get back with needle.
- Pass the cell through a 100um cell strainer with the pipette
- Spin down for 5 mins at 4°C at 1700 rpm
- Rinse the petri dish and the Falcon with medium.
C. cKit enrichment
Cell preparation
- Pool cells from 3 mice into a single samples (this will give 2 samples of 3 mice)
- Centrifuge cells at 1700rpm 5 min 4°C
- Take off the supernatant and leave the last drop
- Add 25 ul of a-CD117 beads in each tube, mix, add 125ul of cold 10%RPMI, mix.
- Incubate for 20 min on ice covered by aluminium
- Add 5ml of cold 10%RPMI to the cells
- Pass through a 100 um cell strainer into 50 ml tube.
- Wash tube and cell strainer with 5 ml of cold medium.
- Take off the last drop of cells under the cell strainer with an 1000ul pipette.
- Centrifuge cells at 1700 rpm 6min 4°C
- Resuspend in 3ml cold medium.
MACS for c-kit+ cells
- Wash the LS column with 3ml of cold 10%RPMI
- Mix cells with an insulin syringe with short 25G needle
- Eject cells against the LS column wall.
- Wash the tube and column with 3 ml of medium. [end of the ckit- collection]
- Detach the column, put into a new 15mL falcon (ckit+ collection tube) and fill up with medium (5mL)
- Insert the plunger and eject the cells [end of the ckit+ collection]
- Count cells
D. Sorting
| Staining for | Sca1(1:200, clone = D7) c-kit (1:100, clone = M1/70) flt3 (1:100, clone = A2F10) CD150 (1:100, clone = TC15-12F12.2) |
| Staining in | 10% RPMI |
| Incubation time | 30 minutes on ice |
| Washing | directly through filter FACS tubes |
| Resupension | in 10% RPMI |
| Expected sorting yield | MPP: 100,000 cells HSC: 10,000-14,000 cells |
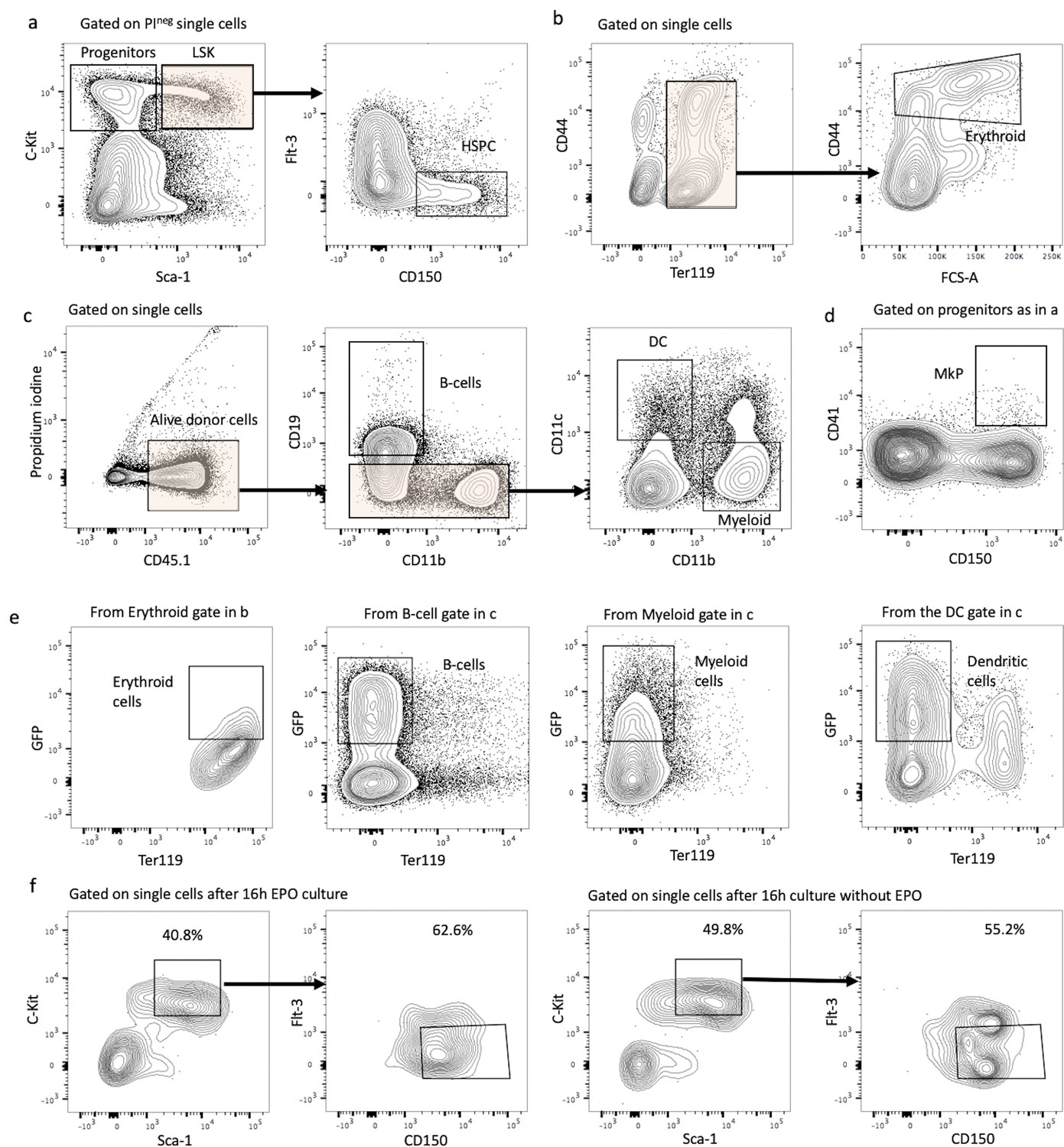
Figure 1: Gating strategies and hematopoietic stem and progenitor cell (HSPC) marker expression after lentiviral transduction and ex vivo culture with or without erythropoietin (EPO). (a) HSPCs were gated as propidium iodide-negative single C-Kit+ Sca-1+ Flt3- CD150+ cells of C-Kit+-enriched bone marrow cells. (b) Erythroblast cells were gated as Ter119+ CD44+ FSChi cells on Ter+ enriched cells. (c) Gating strategy for B-cells (CD19+ CD11b-), dendritic cells (CD19 CD11b- CD11c+), and myeloid cells (CD119- CD11c- CD11b+) on Ter119- live single-donor cells. (d) Gating for MkP (C-Kit+ Sca-1- CD150+ CD41+) from C-Kit+-enriched bone marrow cells. (e) Sort gating for GFP+ erythroid, myeloid, B-, and dendritic cells, respectively, used for barcoding analysis. (f) Representative flow cytometry plots of sorted HSPC pool after 6 hr lentiviral transduction and 16 hr ex vivo incubation with or without EPO.
Step 2-Introducing barcodes-transduction
- Centrifuge the cells and remove the supernatant
- Resuspend in 100uL of Stem Span + 50ng/mL mSCF + 1,3uL of virus corresponding to the LG2.2 lentivirus library. The precise amount of virus was titrated to achieve a transduction efficiency of 10%. This can be reduced to 1% to further reduce multiple barcode usage.
- Mix with the pipette
- Centrifugate at RT 90 min at 300g (brake 2-2)
- Remove the plate and put in the incubator at 37°C 5% CO2 during 4.5h
- Wash twice the cells with complete RPMI
- Resuspend in 500uL of Stem Span + 50ng/mL mSCF
Step 3-EPO treatment
After transduction, cells were incubated with or without human recombinant EPO (Eprex, erythropoietin alpha, Janssen) in Stem Span culture medium supplemented with 50ng/mL mSCF (SS+) at a final concentration of 1000 or 160 ng/ml or PBS for 16 hr at 37°C.
- Coat a 1,5 ml Eppendorf tube with 1 ml 10% RPMI.
- Transfer transduced cells into coated Eppendorf tubes
- Add 1 ml of 10% RPMI, centrifuge 6 min 1700 rpm, 22°C.
- Take off supernatant with 1 ml pipette.
- Resuspend in 400µl SS+ or 420 if transduction control and mix well.
- Take transduction control 20 ul in extra well on plate and add 100 ul or more of SS+.
- Take 200µl for the non-EPO condition and place it into a well.
- Add EPO on the rest and place it into well.
- Culture the cells for 16h at 37°C.
Step 4-injection of barcoded cells into irradiated recipients
After EPO incubation, barcoded MPP2 and unbarcoded CD48- HSPCs were mixed at a ratio of 32/45 (to be as close as possible to the original ratio of both populations in the HSPCs). On average, 2600 cells (mean 2684 cells ± 175 cells) were injected in the tail vein of each mouse in a volume of 100 microliters. Three hours prior to tail vein injection recipient mice (8 week old BL6N D45.1/1 male mice) were 6 Gy sublethally irradiated on a CIXD irradiator.
- Coat a 1,5 ml Eppendorf tube with 1 ml 10% RPMI.
- Mix incubated cells well and transfer into coated Eppendorf tube.
- Wash wells again with 200 ul of 10%RPMI.
- Centrifuge 6 min 1700 rpm 22°C.
- Remove supernatant and wash again in DPBS.
- Resuspend cells in number of mice*100 ul+20 ul and take fraction (1%) for flow cytometry check -> transfer into FACS tubes with 100 ul (or 50ul) antibody mix (as previous day, without CD34) and keep for 35 min (flt3) or 15 (without flt3) in fridge.
- Keep all samples at 4°C till injection or flow cytometry check.
- Perié, L, Cosgrove, J and Eisele, A(2024). HSPC and MPP2 isolation, barcoding, EPO treatment, and transplantation. Bio-protocol Preprint. bio-protocol.org/prep2564.
- Eisele, A. S., Cosgrove, J., Magniez, A., Tubeuf, E., Tenreira Bento, S., Conrad, C., Cayrac, F., Tak, T., Lyne, A., Urbanus, J. and Perié, L.(2022). Erythropoietin directly remodels the clonal composition of murine hematopoietic multipotent progenitor cells. eLife. DOI: 10.7554/eLife.66922
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
