Advanced Search
Cell culture
Last updated date: Mar 20, 2020 Views: 1057 Forks: 0
Primary neuron culture from Drosophila larval brain
This protocol is based on the ones from the Sprecher's lab (Nature protocol. “In vitro imaging of primary neural cell culture from Drosophila”) and Zipursky's (Liming Tan, 40% 25 Retina).
Modified Dissecting Saline
Make 10X Modified Dissecting Saline; filter the 10X stock; add autoclaved dH2O to make 1X Modified Dissecting Saline. Store 10X and 1X at 4°C.
Components |
Final concentration |
10X stock concentration |
Molecular weight | 500 ml 10X stock From Stock solution
| 500 ml 10X stock From powder |
HEPES pH 7.5 |
9.9 mM |
99mM | 260.3 (sodium salt, C8H17N2O4SNa)
| 1M: 49ml
| |
NaCl | 137 mM | 1370 mM | 58.44 | 4M: 170ml
| |
KCl | 5.4 mM | 54 mM | 74.55 | 1M: 27ml
| |
NaH2PO4 | 0.17 mM | 1.7 mM | 137.99 (•H2O) | 100mM: 8.5ml
| |
KH2PO4 | 0.22 mM | 2.2 mM | 136.09 | 100mM: 11ml
| |
Glucose | 3.3 mM | 33 mM | 180.16 | 3.0g | |
Sucrose | 43.8 mM | 438 mM | 342.30 | 75.0g | |
Total | 500ml | ||||
- 1M HEPES pH 7.5 (sodium salt, MW 260.3): 26.0g in 50 ml ultrapure dH2O, adjust pH to 7.5, final volume 100ml.
- NaCl 4M (MW 58.44): 116.9g in 500ml
- KCl 1M (MW 74.55): 14.9g in 200ml
- NaH2PO4(•H2O) 100mM (MW 137.99): 1.4g in 100ml
- KH2PO4 100mM (MW 136.09): 1.4g in 100ml
Supplemented Schneider's medium 20% Fetal bovine serum
5µg/ml Insulin (1:2000 from stock)
100µg/ml Penicillin+100µg/ml Streptomycin (1:500 from stock) 10µg/ml Tetracycline (1:1000 from stock)
10µg/ml Gentamycin (1:1000 from stock)
Liberase stock
(Roche, Liberase TM Research Grade, 05401119001, 2X5 mg) Store the powder at -20°C freezer.
Add 2ml 1X Sterile Modified Dissecting Saline to dilute the liberase into 2.5mg/ml (from 26 Wünsch unit/vial to 13 Wünsch units/ml); aliquot into 20 ul/tube, and store at -20°C.
Before Dissection:
Culture larvae with autoclaved yeast paste (prevents contamination)
Prepare coverslips:
Wash coverslips overnight in chromic acid.
Rinse coverslips 30mins in running distilled water.
Coat coverslips with Concanavalin A (1mg/ml, 30mins-1hr room temperature) Rinse off ConA 10mins in running distilled water.
Dry coverslips. Can be used for at least one month
Sterilise the coverslips by UV on both sides before dissection.
Warm up dissection saline to rtp.
Prepare supplemented Schneiders medium (Make fresh for every neuron prep)
In dissection hood:
- Spray the dissection forceps (we use 2 number 5 forceps), silicone coated dish (dissection dish) and surfaces with 70% EtOH.
- Select third instar larvae from vial with a paintbrush. Sterilise in 70% EtOH for 5mins.
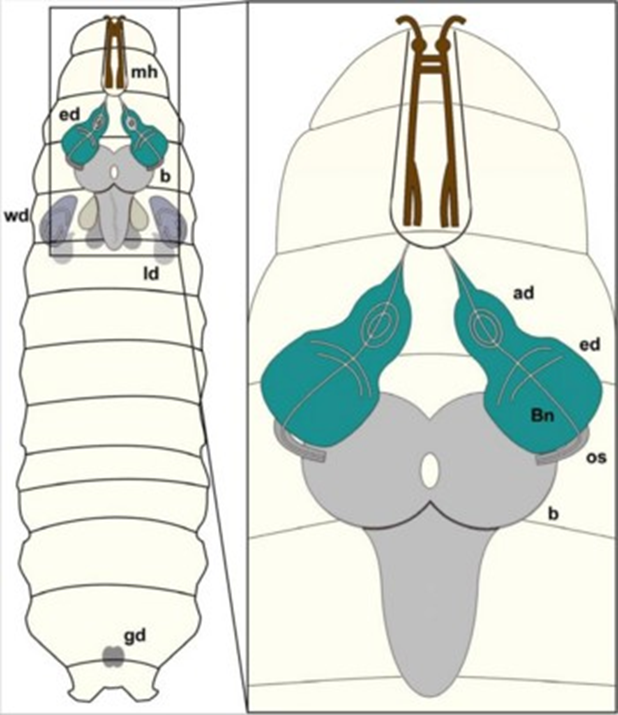
Afterwards leave them in dissection saline. - Transfer larvae individually to dissection dish with 1-2ml dissection medium. tear the larvae apart using a pair of forceps; clean the tissues around the optical lobes plus the ventral ganglion (larval brain, grey part in the following cartoon illustration). Transfer the larval brain into a 1.5ml sterile, silicon coated tube containing 200ul dissection saline.
- Dissect 10-20 brains each time. Dissection should be performed around 1 brain/min.
5. Add 20ul liberase to the larval brains in 200ul dissection saline. Pipette the solution 25-30 times using a siliconized tip.
6. Rotate the tube on the lab rotator for 1 hour; use P200 (set around ~150ul) to pipette the solution 25-30 times every 10-15 min during this period. Use a siliconized/low retention tip. Usually by the end, no large tissues can be seen by eyes.
7. Spin down the cells by centrifuging at 300g (Eppendorf 5415C ~2500rpm) for 5 min.
8. Take out the Liberase solution, and add 1ml supplemented Schneider's medium to stop the reaction; wash the cell pellet and spin down the cells by centrifuging at 300g (Eppendorf 5415C ~2500rpm) for 5 min.
9. Remove medium without touching pellet and add 500ul medium fresh. Repeat spin. Remove excess medium and resuspend with a siliconized tip to give a final concentration of 100ul per 10 brains.
10. Plate 100ul of cells per coverslip.
11. Wait 20 min – 1hr to let the cells attach to the coverslip, and submerge the coverslip with 2ml supplemented Schneider's medium.
12. Culture the neurons in the incubator (25 Celsius, ambient CO2) for a few days to grow neurites. Neurites can be seen within 1 hour.
13. Change medium daily to encourage neurite growth.
- Gelfand, V and Norkett, R(2020). Cell culture. Bio-protocol Preprint. bio-protocol.org/prep254.
- Norkett, R., del Castillo, U., Lu, W. and Gelfand, V. I.(2020). Ser/Thr kinase Trc controls neurite outgrowth in Drosophila by modulating microtubule-microtubule sliding. eLife. DOI: 10.7554/eLife.52009
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
