Advanced Search
Volumetric immunostaining and analysis of sympathetic nerves in tumors
Last updated date: Sep 5, 2023 Views: 137 Forks: 0
Background
Sympathetic nerves in the mammary tumor microenvironment have been shown to regulate tumor growth and metastasis (Kamiya et al. 2019). Using volumetric immunostaining for tyrosine hydroxylase positive sympathetic nerves, we have recently showed that doxorubicin, an anthracycline chemotherapy that is commonly used in the treatment of triple negative breast cancer, increased sympathetic nerve fibers in the 4T1.2 murine mammary tumor model (Chang et al. 2023). The following protocol describes tumor sample preparation, tissue sectioning, immunostaining, clearing of tissue, and imaging acquisition and analysis of sympathetic nerves in the tumor.
Materials and Reagents
- 1x DPBS (ThermoFisher, 14190144)
- 10x DPBS (ThermoFisher, 14200075)
- Tweezer
- Scissors
- 24-well plate
- Cryomold (15 x 15 x 5mm, ProScitech)
- Superglue (Selleys Quick Fix Super Glue)
- Paintbrushes
- Vibratome blade (ProScitech, LO56)
- Parafilm
- 4% PFA (see recipe)
- 1x DPBS with 0.1% (w/v) sodium azide (see Recipes)
- 2% (w/v) agarose (see Recipes)
- SPW buffer (see Recipes)
- Wash buffer (see Recipes)
- FUnGi clearing agent (see Recipes)
- Rabbit anti tyrosine hydroxylase (Merck, clone 152)
- Alexa Fluor 568-conjugated goat anti-rabbit antibody (ThermoFisher, A-11011)
- Hoechst 33342
- Advanced PAP pen (Sigma Aldrich, Z672548)
- Masking tape
- Clear tape
- Microscope slides
- Cover slip
Equipment
- Leica VT1000 S
- Leica SP8 confocal microscope
- Benchtop rocker
- Tube rotator
Procedure
A. Sample Preparation
- Dissect mammary tumor from mice and trim off attached fat and the adjacent lymph node.
- Cut tumor in half.
- Fix tumor in 4% PFA in an Eppendorf tube overnight on a tube rotator.
- Wash tumor with DPBS for 1h on a tube rotator.
- Store tumor samples in 1x DPBS with 0.1% (w/v) sodium azide at 4oC.
B. Sectioning tissue using vibratome
- Prepare 2% (w/v) agarose in DPBS. Ensure agarose is properly dissolved and mixed.
- Set tumor in molten 2% agarose solution in a double stacked cryomold (Figure 1). Remove any bubbles in the agarose and leave on ice.
- Fill 24-well plate with SPW buffer.
- Switch on and set up vibratome according to manufacturer’s protocol.
- Fill buffer tray with ice cold 1x DPBS.
- Remove agarose block from cryomold
- Dry specimen disc and apply a small amount of super glue and gently press agarose block onto it. Ensure the flat surface of the tumor is facing up.
- Once dried, slot specimen disk into buffer tray and screw down with small screw.
- Cut the tumor using the following settings: Thickness: 200 μm, Speed: 8.0, Frequency: 4.0
- Once tumor slice is acquired, use paint brushes to transfer slice into SPW buffer in a 24-well plate and proceed to immunostaining. Optional: Remove agarose attach to the tumor slice.
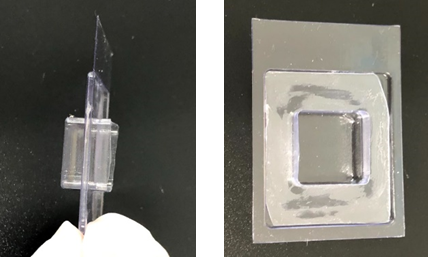
C. Immunostaining and clearing
All the following procedure are done in 24-well plate and at 4oC with agitation using orbital shaker unless stated otherwise. 400 uL of reagents will be sufficient for each well. Tissue slice is transferred using paint brushes.
- Incubate tissue slice in SPW buffer for permeabilization overnight.
- Prepare primary antibody (rabbit anti-tyrosine hydroxylase, clone 152, 1:800) in wash buffer.
- Transfer tissue slice to primary antibody solution. Seal plate with parafilm and incubate for 3 days. For no primary antibody control, tissue slice is incubated with wash buffer.
- After incubation, wash tissue slice in wash buffer for at least an hour each.
- Prepare secondary antibody (AF568 goat anti-rabbit, 1:400) and Hoechst 33342 (1:1000) in wash buffer.
- Transfer tissue slice to secondary antibody solution and incubate overnight.
- After incubation, wash tissue slice in wash buffer twice at least for an hour each.
- Transfer tissue slice to well filled with FUnGi (500uL-1mL) to clear overnight at room temperature.
- Store tissue slice in fridge until imaging.
D. Tissue imaging and analyses
- Clean microscope slide and coverslip with Kimwipe to remove dust particles.
- Add 1-2 layer of masking tape on both end of slide that will be cover by cover slip. This will create enough height so that the coverslip will not press on the tissue when mounted.
- Using PAP pen to create a hydrophobic area in the microscope slide.
- Transfer tissue slice onto the clean microscope slide. Remove any bubble gently using a pipette tip.
- Add a few drops of FUnGi solution on tissue slice.
- Gently place coverslip on top of the tissue.
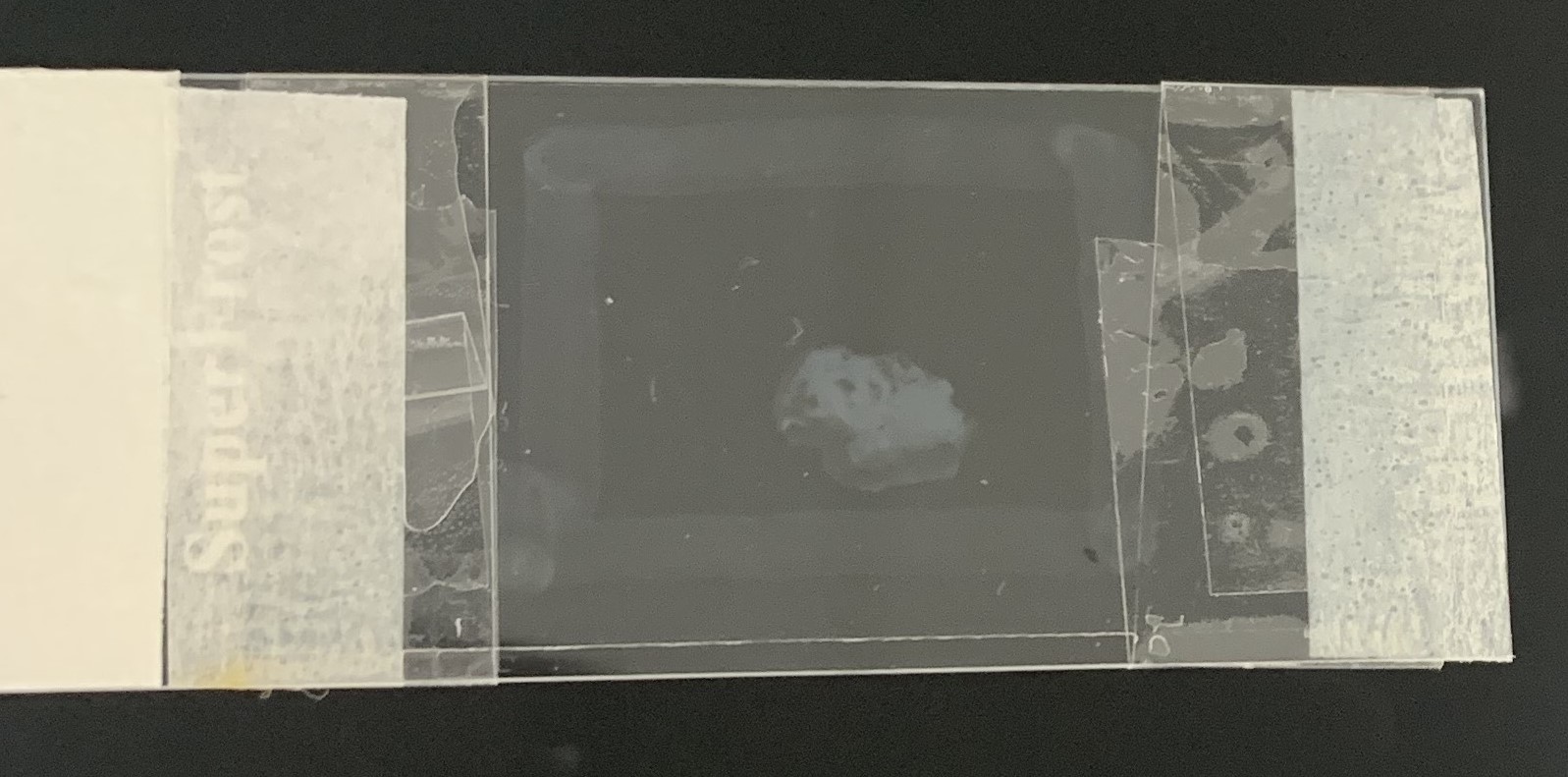
- Tape the coverslip to the slide using a clear tape on both ends to secure the coverslip (Figure 2). Optional: Seal the remaining edges of the slides with nail polish.
- Image the tissue under a Leica SP8 confocal microscope using a 20x Plan Apo 0.75-numerical aperture (NA) objective under resonant mode. Acquire image using the following parameters.
- 16-bit
- zoom: 2
- line average: 4
- Pinhole: 2
- Z- stack size: 88µm
- Z-step size: 4µm
- Merge the images using the LAS X Navigator software.
- Convert image file to Imaris compatible format using ImarisFileConverter software (version 9.3.1).
- Analyse nerve and tumor volume using Imaris software (version 9.7).
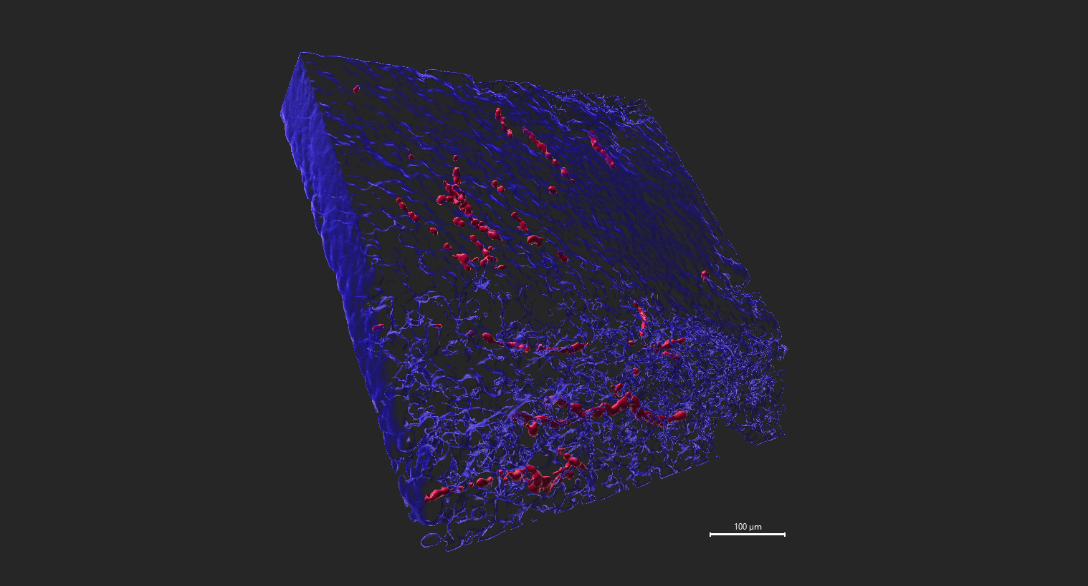
- Create surface for tyrosine hydroxylase positive (TH+) nerves.
- Create surface for tumor tissue using Hoechst 33342 nuclei stain (Figure 3).
- Acquire volume statistic for TH+ nerves and tumor volume.
- Nerve density (%) is expressed as volume of TH+ nerves/volume of tumour tissue x 100% .
Recipes
1. 4% PFA
Weigh 4g of paraformaldehyde. Add 50mL of MilliQ water. Add 1mL of 1M NaOH. Stir gently on a heating block at ~60OC until paraformaldehyde is dissolved. Add 10mL of 10X PBS allow mixture to cool to room temperature. Adjust pH to 7.4 with 1M HCl (~1mL). Adjust final volume to 100mL with MilliQ water. Filter the solution through a 0.45 µm membrane filter to remove any particulate matter. Store aliquot in -20oC for several months.
2. 0.1% sodium azide
Weigh 100mg sodium azide. Add 100mL DPBS. Stir and store at 4oC.
3. 2% agarose
Weigh 2g agarose. Add 100mL DPBS. Microwave the mixture to dissolve agarose. Cool down agarose before using it to embed tumor.
4. SPW buffer (Special Permeabilization Wash buffer)
Weigh 1g BSA and add 50mL 10x PBS, 1mL Tween-20, 1mL Triton X-100 and 1mL 10% SDS. Stir gently until all components are dissolved. Make up the volume to 500mL with MilliQ Water. Filter the solution through 0.45μm filter. Store at 4oC.
5. Wash buffer.
Weigh 1g BSA and add 50mL 10x PBS, 500uL TritonX-100 and 1mL 10% SDS. Stir gently until all components are dissolved. Make up volume to 500mL with MilliQ Water. Filter the solution through 0.45μm filter. Store at 4oC.
6. FUnGi clearing agent
Weigh 26.5g distilled water, 112.6g fructose and 37.6g urea. Stir the mixture gently. Slowly add 157.1g glycerol into the mixture. Wrap the vessel containing the mixture with aluminium foil and leave the mixture stirred overnight. Store the mixture in aluminium foil and at 4oC. Warm up the mixture before use. Note: ensure urea is broken down to small powders to facilitate dissolution.
References
- A. Kamiya, Y. Hayama, S. Kato, A. Shimomura, T. Shimomura, K. Irie, R. Kaneko, Y. Yanagawa, K. Kobayashi, T. Ochiya, Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 22, 1289–1305 (2019).
- A. Chang, E. Botteri, RD. Gillis, L. Löfling, CP. Le, AI. Ziegler, NC. Chung, MC. Rowe, SA. Fabb, BJ. Hartley, CJ. Nowell, S. Kurozumi, S. Gandini, E. Munzone, E. Montagna, N. Eikelis, SE. Phillips, C. Honda, K. Masuda, A. Katayama, T. Oyama, SW. Cole, GW. Lambert, AK. Walker, EK. Sloan, Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med. 15, eadf1147 (2023).
- Chang, A and Sloan, E(2023). Volumetric immunostaining and analysis of sympathetic nerves in tumors. Bio-protocol Preprint. bio-protocol.org/prep2413.
- Chang, A., Botteri, E., Gillis, R. D., Löfling, L., Le, C. P., Ziegler, A. I., Chung, N., Rowe, M. C., Fabb, S. A., Hartley, B. J., Nowell, C. J., Kurozumi, S., Gandini, S., Munzone, E., Montagna, E., Eikelis, N., Phillips, S. E., Honda, C., Masuda, K., Katayama, A., Oyama, T., Cole, S. W., Lambert, G. W., Walker, A. K. and Sloan, E. K.(2023). Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Science Translational Medicine 15(693). DOI: 10.1126/scitranslmed.adf1147
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
