Advanced Search
Isolation of synaptosomes and postsynaptic densities
Last updated date: Sep 1, 2023 Views: 265 Forks: 0
Waxham Lab Protocol to produce synaptosomes, synaptic junctions and post-synaptic densities.
All animal procedures are accomplished under strict guidance of IACUC approved protocols.
Note that all steps should be done at 4oC, and all centrifuge steps with sucrose gradients should be performed with the slow acceleration/deceleration feature to not disturb the gradients. On a Beckman ultracentrifuge this is accel (1) & decel (1).
We typically use forebrains in our preparations, unless targeting other areas, by removing the brain from the cranial cavity in ice cold saline, then make a cut along the fissure between the cerebellum and forebrain and push only the forebrains forward in the following protocol.
Part 1 (Synaptosomal Prep)
- Homogenize brains in buffer A (+leupeptin 1 ug/ml)
- Homogenize 3 brains at a time in 15ml buffer A
- 12 full strokes with drill press driven glass/Teflon homogenizer (0.2 mm clearance)
- Save 100 ul homogenate for diagnostics (if needed)
- Centrifuge homogenate from step 1 for 10 min at 3500 RPM (1.4k x g) in JA20 rotor (2 tubes)
- Save the supernatant.
- Save pellets (2) for re-extraction.
- Pellets are very soft at this point so be careful not to disturb them too much. Carefully pipette the supernatant off the pellet but stop before you begin to aspirate the pellet. Occasionally everything does not spin down, and the top layer of the pellet will look “fluffy”. If this is the case pipette as much supernatant off as possible without aspirating contaminants. The small amount of supernatant that remains will be removed during the next centrifugation.
- Add 25 ml buffer A to each pellet and re-homogenize.
- 5 strokes with homogenizer by hand
- Centrifuge re-homogenized pellets (2) for 10 min at 3500 RPM (1.4k x g) in JA20 rotor
- Save supernatant and combine with supernatant from step 2.
(~100 ml total)
- Discard pellets
- Centrifuge combined supernatants for 10 min at 11,000 RPM (13.8k x g) in JA20 rotor (4 tubes)
- Discard supernatant
- The pellets are moderately tight at this point so you can decant the supe if you are careful. Turn the tube so when you pour the pellet is on the side of the tube facing the bench top. This way you pour the soup off the top of the pellet. Just watch the pellet, because after the supernatant is mostly decanted the pellet will start to flow, at which point stop decanting.
- Save Pellets (4)
- Resuspend each pellet into 10 ml buffer B (+leupeptin 1ug/ml), combine, and homogenize.
- 5 strokes with homogenizer by hand
- Apply homogenate from step 6 to Sucrose Gradient 1 (4 tubes)
Sucrose Gradient 1
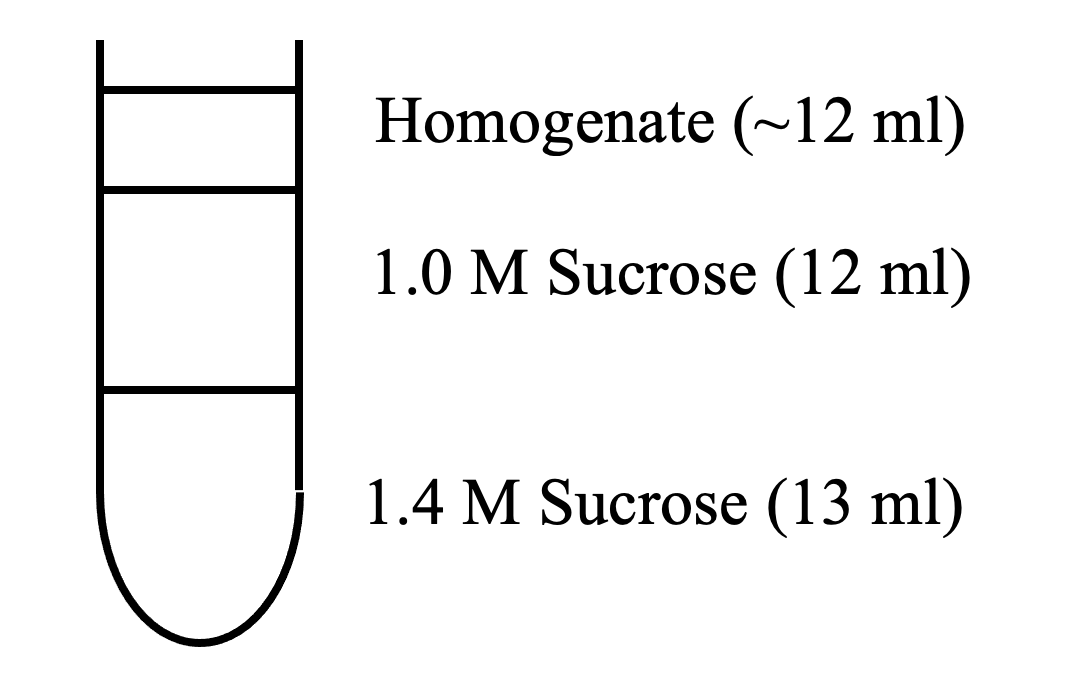
- Centrifuge for 120 min at 25,000 RPM (112k x g) in SW28 rotor
- Remove interface between 1.0 M and 1.4 M sucrose and add to 4 separate 50 ml conical.
- This is the synaptosomal fraction
- Save 100 ul for diagnostic purposes (if needed)
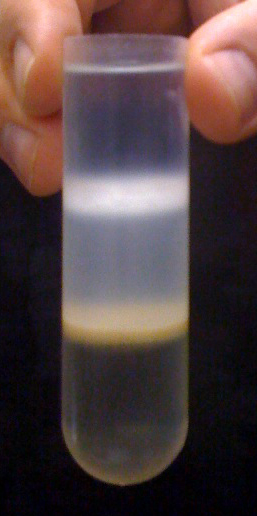
This is what the gradient should look like after centrifugation. The lowest interface (brownish color) is what you want to remove and save for the next step. There is usually a small pellet on the bottom that is not visible in the photo.
*If you only want synaptosomes can stop here and freeze aliquots. If this concentration of sucrose is a problem, can centrifuge the preparation by diluting the sucrose below 0.9M with Buffer “A” and centrifuging in SW28 rotor for 20 min at 16,000 RPM (32.8K x g) at 4oC. Resuspend pellet in buffer of choice.
If further fractionation is desired follow these additional steps.
Part 2 (Synaptic Junction Prep)
- To each conical, add buffer B to 17.5 ml and then add 17.5 ml Triton Extraction Buffer
- Homogenize by hand (~ 3 strokes)
- Use a separate homogenizer when detergents are involved, because detergent contamination in homogenizer may cause problems with the synaptosomes in subsequent preparations.
- Turn homogenate with Triton Extraction Buffer in cold room for 15 min to allow lipid extraction.
- Centrifuge homogenate for 20 min at 16,000 RPM (32.8k x g) in SW28 rotor
- Discard supernatant
- Save pellets (4)
- This is the Synaptic Junction fraction.
- Save 50 ul for diagnostic use (if needed).
- Re-suspend pellets in 2 ml buffer B each and combine (~8 ml)
- Apply re-suspended pellets to Sucrose Gradient 2
Sucrose Gradient 2
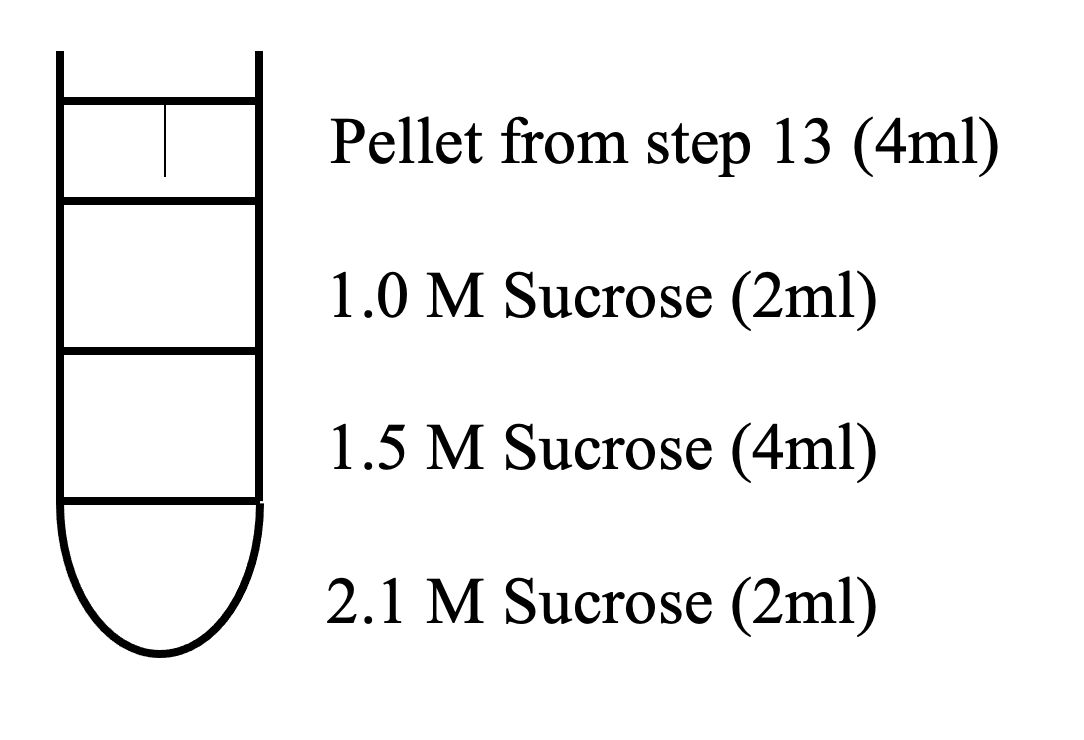
- Centrifuge for 120 min at 40,000 RPM (273k x g) in SW41 rotor.
- Remove interface between 1.5 M and 2.1 M sucrose with PLASTIC pipette.
- Add water to 3 ml and resuspend/hand-homogenize.
- Remove supernatant and discard.
Part 3 (Postsynaptic Density Prep)
- Add 3 ml Triton/KCL Extraction Buffer to bring to a total of 6 ml.
- Rotate for 30 min at 4oC.
- Add to Sucrose Gradient 3
Sucrose Gradient 3

- Centrifuge for 21 min at 35,000 RPM (210k x g) in SW41 rotor
- Remove interface between 1.5 M and 2.1 M sucrose and adjust to 5 ml with H2O (mix well)
- Centrifuge for 15 min at 20,000 RPM in SW55 rotor
- Add 500 µl of 20% Glycerol/5mM Hepes-KOH, pH 7.4 to pellet.
- Resuspend and store in 25ul aliquots at -80oC.
*Note PSDs are extremely sticky. Avoid glass surfaces and pipets.
Reagents needed for one prep:
Sucrose for gradients
1.0 M 4(12ml) + 2(2ml) = 52 ml (make 70)
1.4 M 4(13ml) = 52 ml (make 70)
1.5 M 2(4ml) + 2(4ml) = 16 ml (make 25)
2.1 M 2(2ml) + 2(2ml) = 8 ml (make 12)
Buffer A = 2(50ml) = 100 ml
Buffer B = 40 ml + 4(17.5ml) + 4(2ml) = 118 ml
Triton Extraction Buffer = 4(17.5ml) = 70 ml
Triton/KCL Extraction Buffer = 3ml
Solutions for PSD prep:
Sucrose Solutions (sucrose MW = 342.30 g/m):
1) 1.0 M 23.96 g sucrose into 70ml* of H20 0.7 ml of 1 M HEPES/KOH pH 7.4
2) 1.4 M 33.55 g sucrose into 70ml* of H20 0.7 ml of 1 M HEPES/KOH pH 7.4
3) 1.5 M 12.84 g sucrose into 25ml* of H20 0.25 ml of 1 M HEPES/KOH pH 7.4
4) 2.1 M 8.63 g sucrose into 12ml* of H20 0.12 ml of 1 M HEPES/KOH pH 7.4
* These are final volumes so make sure to start out with a smaller volume of water and finish with these volumes.
Filter and store at 4 degrees
Buffer A:
Final Concentrations For 150 ml Final Volume
5 mM HEPES/KOH, pH 7.4 750 uL of 1.0 M HEPES/KOH, pH 7.4
0.32 M sucrose 16.4 g of Sucrose
1 mM MgCl2 150 uL of 1.0 M MgCl2
0.5 mM CaCl2 75 uL of 1.0 M CaCl2
1 ug/ml Leupeptin 3 uL of 50 mg/ml leupeptin in water
bring to150 ml final volume, filter, store at 4oC.
Buffer B:
Final Concentrations For 150 ml Final Volume
5 mM HEPES/KOH, pH 7.4 750 uL of 1.0 M HEPES/KOH, pH 7.4
0.32 M sucrose 16.4 g of Sucrose
1 ug/ml Leupeptin 3 uL of 50 mg/ml leupeptin in water
bring to 150 ml final volume, filter, store at 4oC.
Triton Extraction Buffer:
Final Concentrations For 100 ml Final Volume
5.0 mM HEPES/KOH, pH 7.4 500 uL of 1.0 M HEPES/KOH, pH 7.4
0.32 M sucrose 10.9 g of Sucrose
1% TX-100 5 ml of 20% TX-100 in water
Triton/KCL Extraction Buffer:
Final Concentrations For 10 ml Final Volume
5.0mM HEPES/KOH, pH 7.4 50uL of 1.0 M HEPES/KOH, pH 7.4
1% TX-100 0.5 ml of 20% TX-100 in water
150 mM KCl 375 uL of 4 M KCl
- Waxham, M and Levental, I(2023). Isolation of synaptosomes and postsynaptic densities. Bio-protocol Preprint. bio-protocol.org/prep2408.
- Tulodziecka, K., Diaz-Rohrer, B. B., Farley, M. M., Chan, R. B., Paolo, G. D., Levental, K. R., Waxham, M. N. and Levental, I.(2016). Remodeling of the postsynaptic plasma membrane during neural development. Molecular Biology of the Cell 27(22). DOI: 10.1091/mbc.e16-06-0420
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
