Advanced Search
Immunofluorescence, immunoprecipitation, and ELISA using the Scicons anti-dsRNA J2 antibody
Last updated date: Jun 22, 2023 Views: 972 Forks: 0
TRIzol-based RNA extraction for Drosophila melanogaster – For use with ELISA
- Extract RNA with TRIzol by adding a total volume of 500µL TRIzol to six fly heads
- Homogenize fly heads first with 50µL, then adjust to 500µL.
- Spin 10 minutes 4°C 12kXg
- Incubate supernatant five minutes at room temperature
- Add 100µL chloroform and vortex for at least 30 seconds. Let sit for three minutes and then spin at 4°C for 15 minutes at 12kXg
- Transfer upper phase to a fresh tube
- Add 250µL isopropanol, incubate ten minutes at room temperature
- Spin for 15 minutes at 4°C 16kXg
- Wash the pellet in 500µL 75% ethanol three times. Spin down at 4°C for five minutes between each wash.
- Air dry 30 minutes
- Resuspend in 15µL RNAse/DNAse free water and measure concentration.
- If using RNA for downstream applications such as sequencing, adjust elution concentration as needed to increase or decrease minimum input volumes and concentrations. Using a more sensitive method like a Qubit high sensitivity RNA kit is recommended.
Double stranded RNA immunoprecipitation with J2 – Drosophila melanogaster, mouse or human brain tissue
DAY 1 – Input preparation
Buffers:
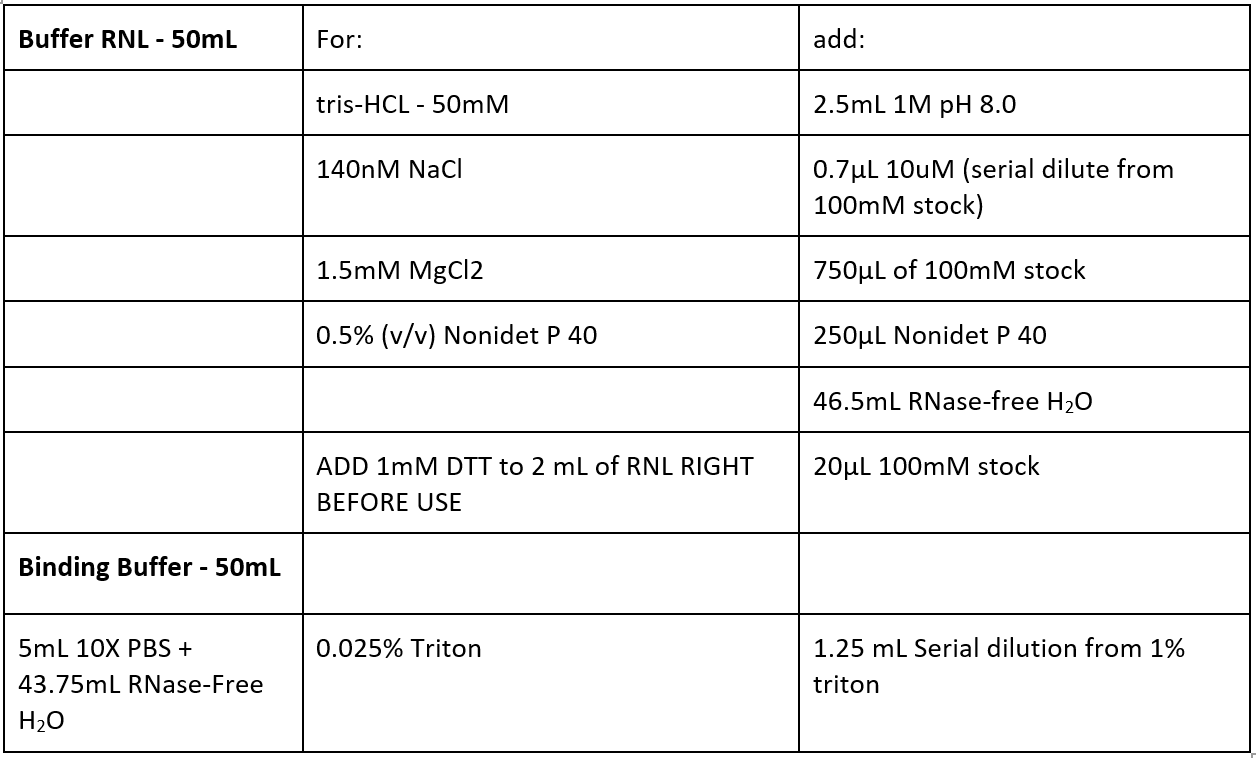
- Isolate 50-100mg of frozen tissue or fly heads
- 60 heads from flies
- One ice – lyse and homogenize with blue homogenization stick in 200µL RNL + 1µL RNaseOUT for ten minutes
- First lyse in 50µL RNL, then add 150µL RNL
- Centrifuge samples for 2 minutes at max speed → make sure this is done at least at 4°C.
- Pre-wash with 40µL protein A beads
- Wash beads 3x5 minutes with binding buffer while rotating
- Incubate supernatant with 40µL pre-washed protein A beads for one hour at 4°C while rotating
- Split incubated supernatant from step four into two parts:
- Combine your antibody (for dsRNA IP, use 5µg of J2) with binding buffer + 100µL pre-cleared lysate from step 4 to a total volume of 200µL. This is the immunoprecipitated sample. Rotate overnight at 4°C.
- For isotype control: Combine isotype control (5µg) + 100µL pre-cleared lysate from step 4 to a total volume of 200µL. Rotate overnight at 4°C.
- Save the remaining portion of the pre-cleared lysate as “input” at -80C
DAY 2 – IP and dsRNA isolation
I. Immunopreciptate J2-bound dsRNA by incubating with 50µL of prewashed Dynabeads (protein A)
- Pre-wash 50µL beads per sample for 3 x 5 minutes with COLD binding buffer
- Incubate lysate + antibody with beads for three hours at 4C while rotating
- Remove lysate
- Wash beads 5 x 5 minutes with COLD binding buffer
II. dsRNA extraction
- Extract dsRNA with TRIzol by adding 500µL TRIzol to beads from part I. Ice for 5 – 10 minutes
- Spin samples for 10 minutes 4°C 12kxg.
- Incubate supernatant 5 minutes at room temperature.
- Add 100µL chloroform and vortex. Let sit for 3 minutes and then spin at 4°C for 15 minutes at 12kxg.
- Transfer upper phase to fresh tube.
- Add 1µL glycogen to each sample as a carrier for dsRNA
- Add 250µL isopropanol, incubate ten minutes at room temperature
- Spin for 15 minutes at 4°C 16kxg
- Wash the pellet in 500µL 75% ethanol three times. Spin down at 4°C for five minutes between each wash.
- Air dry pellet for 30 minutes
- Resuspend in 15µL RNAse/DNAse free water
- Measure concentration using a Qubit high sensitivity RNA kit
ELISA DSRNA J2 AND K2 with Drosophila Melanogaster Brain Tissue
DAY 0 (or at least a few days before) – clean glassware and troughs with RNAse Away and autoclave
DAY 1 – COATING PLATE WITH J2 AND TOTAL RNA ISOLATION
- Make fresh sodium phosphate buffer 0.1M pH 6.5

2. Let sodium phosphate buffer chill for about five minutes on ice, then mix with J2 antibody to a final concentration of 1µg/mL. 100µL/well.
3. Incubate plate with optical cover at 4C overnight
4. Isolate total RNA from samples with TRIzol
DAY 2 – ELISA SAMPLE CAPTURE AND DETECTION
- Prepare wash buffers:
- Wash buffer 1 - 1X PBS 0.5% Tween 20 – at least 500mL
- 2.5mL Tween 20
- 50mL 10X PBS (RNAse-free)
- 447.5mL H2O
- Wash buffer 2 - 1X TBS Tween 20 – at least 50mL
- 200µL Tween 20
- 20mL 10X TBS (RNAse-free)
- 179.8mL H2O
- Wash buffer 1 - 1X PBS 0.5% Tween 20 – at least 500mL
- Wash plate 3X5 minutes with wash buffer 1
- Dilute samples to 300µL by adding 1X PBS (RNAse-free)
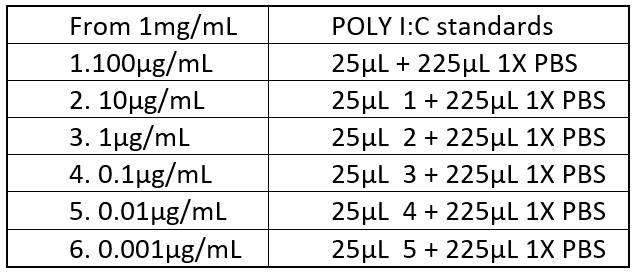
- Create poly (I:C) standards:
5. Add standards and samples to plate after completing washes
6. Incubate plate for one hour on a rocker at room temperature
7. Wash plate 3 x 5 minutes with wash buffer 1
8. Mix capture antibody K2 1:4
9. Add capture antibody to plate and incubate one hour on a rocker at room temperature
10. Wash plate 3X5 minutes with wash buffer 1
11. Prepare secondary antibody anti-mouse IgM-AP 1:5000 in diH2O
12. Add secondary antibody to plate and incubate for one hour on a rocker at room temperature
13. Remove P-nitrophenyphosphate stock from 4°C so it can equilibrate to room temperature
14. Wash plate 4 x 5 minutes with wash buffer 2
15. During final wash mix P-nitrophenyphosphate in diH2O to 100µg/well (100µL/ well)
16. Keep P-nitrophenyphosphate substrate in the dark until ready to add
17. Add P-nitrophenyphosphate substrate without adding bubbles and place into plate reader for reading at 405nm over two hours every ten minutes. Alternatively, wait 90 minutes and add a stopping solution.
J2 Immunofluorescence – Dissected whole fly brain
- Dissect adult fly brain in 1X PBS, place on slide, cover with coverslip and place on dry ice. After frozen, flip off coverslip for subsequent fixation.
- Fix tissue on slide with 4% PFA for ten minutes
- Wash tissue once with diH2O, and place in cold 1X PBS
- Can store tissues on slides at 4C while collecting remaining samples
- Block in 2% nonfat milk, BSA or goat serum for 30 minutes at room temperature
- Remove from blocking solution and use a hydrophobic pen to encapsulate samples on slide
- Incubate overnight at 4°C with primary antibody (20-100µL volume) in 2% Nonfat milk, BSA or goat serum 1X PBSTr (PBS + 0.3% Triton) in a humidifying chamber filled with diH2O
- Wash 3 x 5 minutes with 1X PBS
- Incubate secondary antibody in 2% BSA or goat serum 1X PBSTr for two hours at room temperature while rocking
- Wash 3 x 5 minutes with 1X PBS
- Mount tissue onto slide and coverslip with Fluoromount G + DAPI
J2 Immunofluorescence – Free floating mouse tissue
1. Wash sections for 3 x 5 minutes with 1X PBS
2. Block in 2% BSA or 2% goat serum for 30 minutes at room temperature
3. Incubate overnight at 4C with primary antibody in 2% BSA or goat serum 1X PBSTr while rocking
a. 24 or 48 well plate can be used. 200µL per well will cover the section. Multiple sections can be incubated in the same well.
4. Wash 3 x 5 minutes with 1X PBS
5. Incubate secondary antibody (1:200) in 2% BSA or goat serum 1X PBSTr for two hours at room temperature while rocking
6. Wash 3 x 5 minutes with 1X PBS
7. Mount tissue onto slide and place coverslip with three drops Fluoromount G + DAPI
J2 Immunofluorescence – Frozen human brain tissue
- Remove cryosection from -80°C and immediately fix in 4% PFA for ten minutes.
- Wash 1X with diH2O
- If UV photobleaching to remove lipofuscin autofluorescence:
- Submerge slide in petri dish (lid covered to prevent light from escaping) filled with 1X sodium citrate 0.05% sodium azide
- 0.5g sodium azide
- 100mL 10X sodium citrate
- H2O to 1L
- Place petri dish on UV light (4°C cold room) with slots aligned to the slide. Top off any spilled sodium citrate, plug in light and bleach for 4.5 hours.
- Can leave overnight, but this will not change the bleaching intensity.
- Unplug light, remove dishes and wash slides once in 1X PBSTr
- Submerge slide in petri dish (lid covered to prevent light from escaping) filled with 1X sodium citrate 0.05% sodium azide
- Block in your preferred blocking solution for 30 minutes at room temperature
- 2% BSA
- 2% milk – recommend placing slides at 4°C if primary antibody is incubated overnight
- 5% goat serum
- Incubate slides with primary antibody J2 1:300 overnight in blocking solution
- 300µL covers one slide, but a hydrophobic pen can be used to circle the sample and reduce needed volume
- Wash twice with 1X PBSTr, once with 1X PBS
- Incubate slides in corresponding secondary antibody 1:200 for two hours at room temperature in blocking solution
- Wash twice with 1X PBSTr, once with 1X PBS
- If utilizing Sudan Black in addition to or instead of UV photobleaching:
- 0.7% SBB in 70% EtOH for ten minutes
- Wash 3 x 5 minutes with with PBSTr
- Mount with Fluoromount G + DAPI and coverslip
- Ochoa, E and Frost, B(2023). Immunofluorescence, immunoprecipitation, and ELISA using the Scicons anti-dsRNA J2 antibody. Bio-protocol Preprint. bio-protocol.org/prep2344.
- Ochoa, E., Ramirez, P., Gonzalez, E., De Mange, J., Ray, W. J., Bieniek, K. F. and Frost, B.(2023). Pathogenic tau–induced transposable element–derived dsRNA drives neuroinflammation. Science Advances 9(1). DOI: 10.1126/sciadv.abq5423
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
