Advanced Search
Collection of human gonadal cells
Last updated date: Mar 13, 2023 Views: 427 Forks: 0
This protocol enables purification of male and female human primordial germ cells (hPGCs) between week 5.5 and 10 of development.
The protocol was adapted from Tang et al. 2015 (PMID: 26046444)
Dissection of human urogenital ridges and staining of gonadal cells
- Urogenital ridges were dissected from the embryo in dissection medium. Subsequently the genital ridge was separated from the mesonephros using a BD Microlance needle (25G x 5/8”) as blade (Figure 1). The mesonephric cells were used as a negative control during the isolation of hPGCs by FACS.
- Genital ridges and mesonephroi were washed with 5ml PBS in 3 cm sterile cell culture dishes and transferred with about 30 µl of PBS into 1.5 ml tubes.
- Both tissues were digested in 300 µl Collagenase digestion mix through incubation at 37°C for 20 to 40 minutes (until the tissue disintegrated). Samples were pipetted up and down every 5-10 minutes to facilitate tissue dissociation.
- The digestion was stopped by adding 1 ml FACS sorting buffer and mixing by pipetting up and down.
- Cells were pelleted by centrifugation at 500 g for 5 minutes at room temperature in a swing-out rotor.
- Supernatant was carefully removed, and cells were resuspended in 90 µl FACS sorting buffer.
- The following antibodies were added to the cells:
- 5 µl of Alexa Fluor 488-conjugated anti-human Alkaline Phosphatase (Clone B4-78) (BD Pharmingen 561495)
- 5 µl of APC-conjugated anti-human CD117 /cKit (clone 104D2) (Invitrogen CD11705)
- The cell suspension was mixed by pipetting up and down and incubated for 15 minutes at room temperature rotating at 10 rpm in the dark.
- 1 ml FACS sorting buffer was added to the cell suspension and mixed by inverting the tubes several times.
- Cells were pelleted by centrifugation at 500 g for 5 minutes at room temperature in a swing-out rotor.
- The supernatant was removed, and cells were resuspended in 400 µl FACS sorting buffer.
- Cells were filtered into a 5 ml polypropylene tube using a 35 µm cell strainer.
- Tubes were kept on ice in the dark until FACS.
FACS of hPGCs and gonadal somatic cells
- Gonadal and mesonephric cells were sorted on a SH800Z Cell Sorter (Sony) using a 100 μm microfluidic sorting chip (Figure 2).
- To confirm the purity, the first 100 hPGCs and gonadal somatic cells were sorted on a Poly-L-Lysine coated microscopy slide (Thermo Fisher Scientific). Cells were incubated on the cover slide in a humidified chamber for at least 20 minutes and processed further after the end of the sorting.
- Cells were directly sorted into 50 µl extraction buffer (PicoPure RNA Isolation Kit, Applied Biosystems) for RNA extraction or 50 µl PBS, 3% fetal calf serum for analysis by ChIP-seq or Western Blot.
- Cells were pelleted by centrifugation at 500 g for 5 minutes at room temperature in a swing-out rotor.
- For RNA extraction, additional 30 µl extraction buffer (PicoPure RNA Isolation Kit, Applied Biosystems) were added to the cell lysate, mixed through pipetting, snap frozen on dry ice, and stored at -80°C until RNA purification. For ChIP-seq, supernatant was removed and cells were gently resuspended in 20 µl chilled (4°C) Nuclei EZ Storage Buffer (Nuclei EZ prep nuclei isolation kit, Sigma) and stored at -80°C. For Western Blot analysis, supernatant was removed and 200 µl PBS was added without disturbing the cell pellet. Supernatant was removed after centrifugation (repeat step 4), and cells were stored in 10 µl PBS at -80°C.
- Cells sorted onto the cover slide (step 2) were fixed with 4% Paraformaldehyde for 15 minutes at room temperature.
- Cells were washed with PBS and Alkaline phosphatase staining was performed using the Leukocyte Alkaline Phosphatase Kit (Sigma) to determine the purity of hPGCs. Staining was evaluated on an inverted microscope and AP positive cells were counted. Only samples with >97% AP positive cells were used for downstream analysis.
Media and buffers
- Dissection medium: DMEM (4.5 g/l glucose, 4 mM L-glutamine; Gibco), 10% fetal calf serum, 1 mM Sodium Pyruvate, 100 U/ml penicillin-streptomycin. Can be stored at 4 °C for up to 4 weeks.
- Collagenase digestion mix: 2.6 mg/ml Collagenase IV in DMEM/F-12, 10 U/ml DNase I. The digestion mix should always be prepared freshly. After dissolving 2.6 mg Collagenase IV (Sigma C5138) in 1 ml DMEM/F-12 (Gibco 21331020) and addition of 10 U/ml DNase I, the digestion mix was incubated for 5 minutes at 37 °C and filtered (0.22 µm sterile syringe filter).
- FACS sorting buffer: PBS, 3% fetal calf serum, 5 mM EDTA
Materials
- BD Microlance needle (25G x 5/8”)
- 3 cm sterile cell culture dish
- Poly-L-Lysine coated microscopy slide
- 5 ml polypropylene tube
- 35 µm cell strainer
- 0.22 µm sterile syringe filter and 1 ml sterile syringe
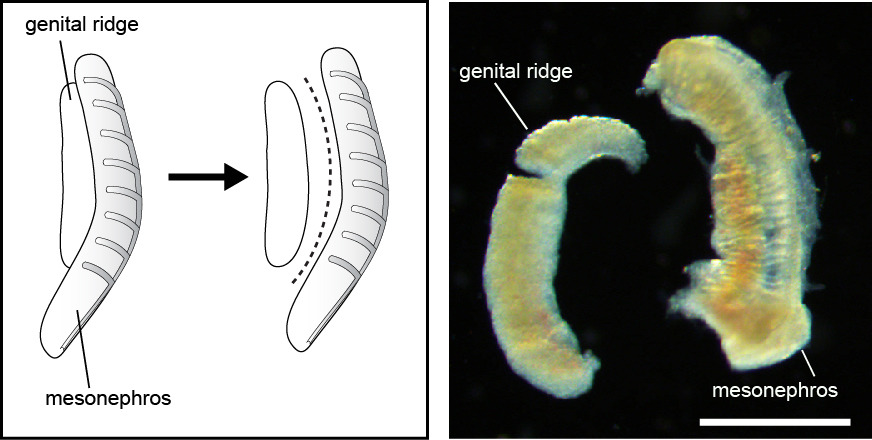
Figure 1: Schematics of the separation of genital ridge and mesonephors (left panel). Dissected week 8 female genital ridge and mesonephros (right panel). Scale bar = 1 mm.
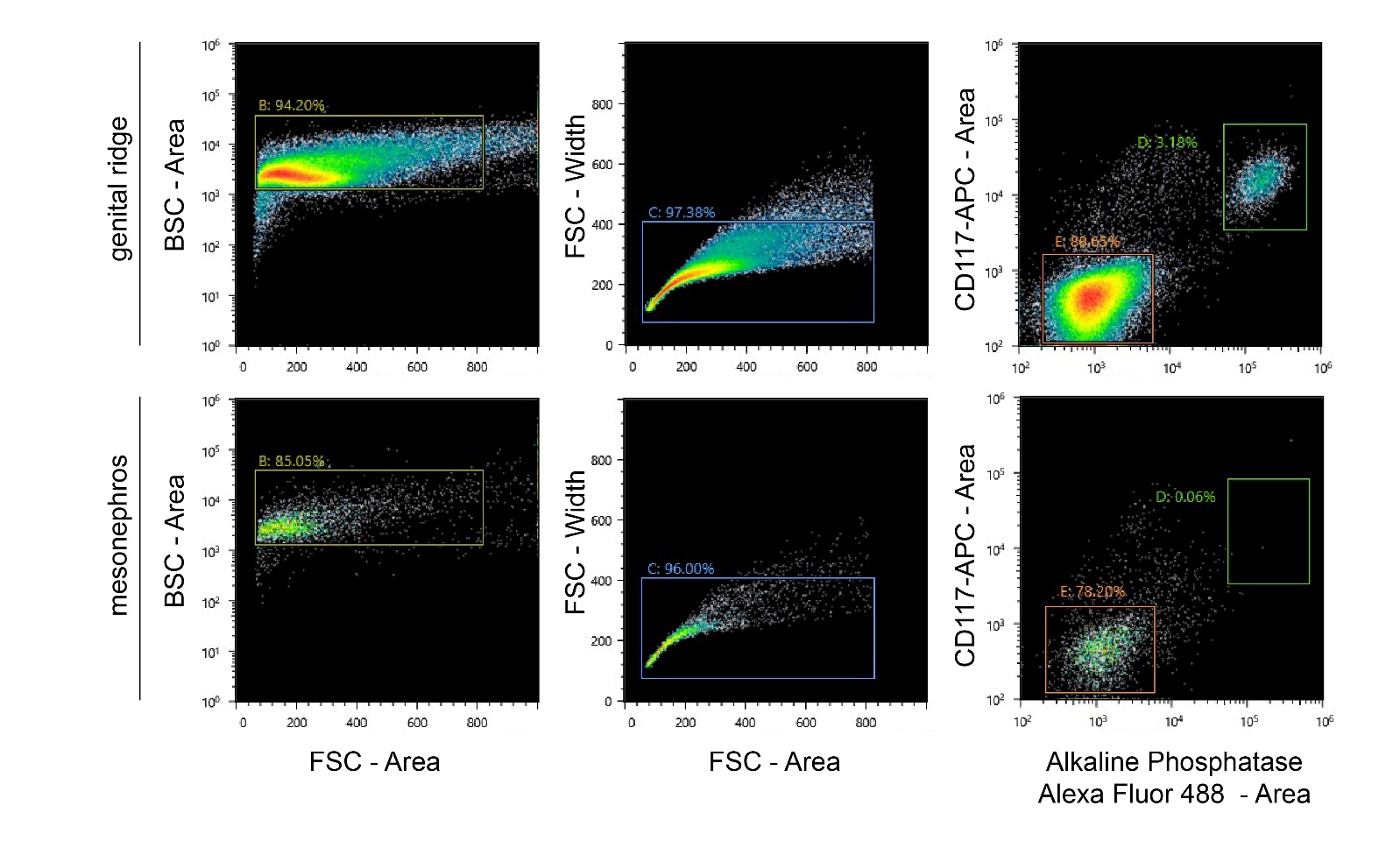
Figure 2: FACS on genital ridges (upper panels) and mesonephroi (lower panels).
- Gruhn, W H, Tang, W W and Surani, M A(2023). Collection of human gonadal cells. Bio-protocol Preprint. bio-protocol.org/prep2173.
- Gruhn, W. H., Tang, W. W., Dietmann, S., Alves-Lopes, J. P., Penfold, C. A., Wong, F. C., Ramakrishna, N. B. and Surani, M. A.(2023). Epigenetic resetting in the human germ line entails histone modification remodeling. Science Advances 9(3). DOI: 10.1126/sciadv.ade1257
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
