Advanced Search
In vitro kinase assay using immunoprecipitated Cdk5 from SCN
Last updated date: Feb 20, 2020 Views: 1271 Forks: 0
SCN extraction
-Mice 2 months old were kept under light: dark (12:12) conditions.
-7 mice for each time point corresponding to ZT 0, 4, 8, 12, 16, 20 (0-12 light on, 12-20 light off) were sacrificed and brain extracted. During the night phase, killing and tissue extraction was performed under red light.
-Brains were frozen in N2 liquid and pooled together for each appropriate time point and samples stored at -80°C.
-The Optic chiasm was removed using forceps and SCN extracted from the Brain.
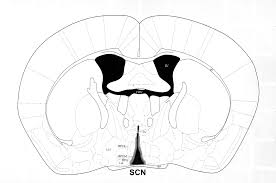
A B
A (Image taken Easton at al., 2004). B The mouse brain library
-7 SCN for each time point were pooled together in the same tube and left on ice or stored at -80°C.
Protein Extraction
-Brain Lysis Buffer + protease inhibitors (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.25% SDS, 0.25% sodium deoxycholate, 1 mM EDTA) was added to the frozen samples (2 times the tissue volume) and samples were homogenized using the blue pestle.

(Image taken from Universal Medical)
-Samples were vortexed and put on ice for 20’ (samples were vortexed each 5’)
-Samples were sonicated for few seconds at 10-30 % of maximal amplitude, in order to eliminate all the residual pieces of tissue not homogenized.
-Samples were centrifuged at 4°C for 20’ at 12000 x g and supernatant was collected (total protein extract).
-Protein were quantified using Pierce Rapid Gold BCA Protein Assay Kit (Thermo fisher) and finally quality of extract was evaluated running samples on a SDS/PAGE 10% gel stained with Coomassie Blue.
-Subsequently the accumulation of the protein of interest (CDK5) around the clock was detected by Western Blot (Rabbit anti-CDK5 cell signaling 1:1000 over night at 4°C) and compared to loading control protein (i.e. Tubulin; Rabbit anti-tubulin 1:1000 Abcam 3 hours Room Temperature)
Immunoprecipitation of CDK5 around the clock
-A protein amount corresponding to between 400 and 800 µg of total extract for each time point discussed before was diluted with Brain Lysis Buffer in a final volume of 250 µL.
-5 ul of CDK5 antibody were added to the mix (antibody ratio 1:50).
-Immunoprecipitation reaction was performed over night at +4°C on a rotary shaker.
-The day after, samples were captured with 80 µL of 50% (w/v) protein-A agarose beads (Roche) and the reaction was kept at 4°C for 3 hours on a rotary shaker. Prior to use, beads were washed three times with the appropriate protein buffer and resuspended in the same buffer (50% w/v).
-The beads were collected by centrifugation (1000 x g) and washed three times with NP-40 buffer (100 mM Tris-HCl pH7.5, 150 mM NaCl, 2 mM EDTA, 0.1% NP-40). After the final wash, each sample was split in 2 different tubes (circa 40 ul of beads for each tube called “tube IP” and “tube KA”, acronyms for Kinase Assay.
IP for Western Blot
-Samples from “tube IP” were resuspended in 2% SDS 10%, glycerol, 63 mM Trish-HCL pH 6.8 and proteins were eluted for 15 min at RT.
-Laemmli buffer was finally added, samples were boiled 5 min at 95°C and loaded onto 10% SDS-PAGE gels.
-Western Blot was performed (Rabbit anti-CDK5 cell signaling 1:1000 over night at 4°C) to detect the amount of CDK5 immunoprecipitated at each time point.
In Vitro Kinase assay
-Reaction was performed adding to samples from “tube KA” a mix containing 5X Kinase buffer ( 30 mM Hepes pH 7.2, 10 mM MgCl2, 1 mM DTT in 25ul of reaction, so that the final concentration is 1X), 1 µg of histone H1 (Sigma-Aldrich), [γ-32P] ATP (10 Ci) ( final volume mix 20 ul + beads), and incubating the samples at 30°C for 1 Hour
-Reaction was stopped with laemmly buffer and samples were boiled at 90°C.
-Samples were loaded on a 15% SDS/PAGE.
-Gel was stained 10’ in Coomassie Blue (20% methanol, 10% Acetic Acid, Coomassie blue)
-Gel was distained in 20% Methanol, 10% Acetic Acid
-Gel was washed 3 times with distilled water
-Gel was equilibrated in drying solution containing 20% Methanol, 10% Glycerol for 1 Hour.
-Gel was dried in a specific gel dryer at 80°C for 1h 30’ (vacuum pump active).
- Finally, the phosphorylation was detected by autoradiography.
Kinase assay evaluation
- CDK5 IP signal obtained from the WB was quantified by optical densitometry (Imaje J / Fijj)
- Phosphorylation signal obtained from the In vitro Kinase assay was quantified in the same way
- The ratio CDK5 IP / [γ-32P] ATP gives the final quantification about the effective kinase activity for each time point.
- Brenna, A and Albrecht, U(2020). In vitro kinase assay using immunoprecipitated Cdk5 from SCN. Bio-protocol Preprint. bio-protocol.org/prep216.
- Brenna, A., Olejniczak, I., Chavan, R., Ripperger, J. A., Langmesser, S., Cameroni, E., Hu, Z., De Virgilio, C., Dengjel, J. and Albrecht, U.(2019). Cyclin-dependent kinase 5 (CDK5) regulates the circadian clock. eLife. DOI: 10.7554/eLife.50925
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
