Advanced Search
Observation and motility assay of zebrafish spermatozoa
Last updated date: Nov 18, 2019 Views: 1332 Forks: 0
Abstract
Malfunctions of motile cilia/flagella often cause primary ciliary dyskinesia (PCD), whose symptoms include male sperm infertility. Zebrafish is a small freshwater teleost and have many desirable features as a vertebrate model. Here we performed observation and motility assay of zebrafish spermatozoa using high-speed camera, which enabled us to describe detailed sperm phenotypes of zebrafish with mutations in PCD-causative genes.
Background
Motile cilia/flagella are hair-like organelles that project from various types of eukaryotic cells. Beating motions of cilia/flagella are required for the locomotion of spermatozoa and the generation of extracellular fluid flow in various organs such as trachea, fallopian tube, and left-right organizer (node; also known as Kupffer’s vesicle in teleosts). PCD is an inherited disorder caused by malfunctions of motile cilia/flagella and characterized by male infertility, recurrent respiratory infections, and inversion of visceral laterality (Knowles et al., 2013). Zebrafish (Danio rerio) is a good model organism for vertebrate genetics with many desirable features, including transparent embryonic body which enables us to observe internal ciliated organs directly, and easy spermatozoa collection without killing male zebrafish. Moreover, recent genome-editing techniques expanded zebrafish potential in reverse genetics. In this paper, we describe observations and motility assays of zebrafish spermatozoa using high-speed camera. Not only CASA (computer-assisted sperm analysis), which can evaluate sperm swimming performances, but also beating frequency analysis and waveform analysis of mutant sperm flagella were performed to obtain insights about the functions of PCD-causative genes.
Materials and Reagents
Sodium Chloride (NaCl) (Nacalai Tesque, catalog number: 31319-45)
Potassium Chloride (KCl) (Nacalai Tesque, catalog number: 28514-75)
di-Sodium Hydrogenphosphate (Na2HPO4) (Nacalai Tesque, catalog number: 31801-05)
Potassium Dihydrogenphosphate (KH2PO4) (Nacalai Tesque, catalog number: 28721-55)
Calcium Chloride Dihydrate (CaCl2·2H2O) (Nacalai Tesque, catalog number: 06731-05)
Magnesium Sulfate Heptahydrate (MgSO4·7H2O) (Nacalai Tesque, catalog number: 21003-75)
Sodium Hydrogen Carbonate (NaHCO3) (Nacalai Tesque, catalog number: 31213-15)
Albumin, from bovine serum (BSA) (Sigma-Aldrich, catalog number: A9418-50G)
24x60mm coverslip (Matsunami, catalog number: C024601)
18x18mm coverslip (Matsunami, catalog number: C218181)
Double-sided tape, 30µm thickness (Kyodo Giken Chemical, catalog number: 200A30)
Note: We found that our paper “Systematic studies of all PIH proteins in zebrafish reveal their distinct roles in axonemal dynein assembly” contains a wrong description about the double-sided tape spacer.
Original: 10 µm spacers (200A10; Kyodo giken chemical)
Correction: 30 µm spacers (200A30; Kyodo giken chemical)
Equipment
inverted microscope (Leica, model: DMI6000B)
high-speed camera (Ditect, model: HAS-L1)
Software
ImageJ
ImgaeJ plug-in, Computer Assisted Sperm Analyzer (Wilson-Leedy and Ingermann, 2007)
Adobe Illustrator
Procedure
>Collection of zebrafish spermatozoa
For detailed protocol, see THE ZEBRAFISH BOOK, Chapter 2.
Anesthetize male zebrafish in tricaine.
Rinse in fish-water, and gently blot the body with a Kimwipe.
Gently squeeze the sides of the fish to expel the spermatozoa from the genital pore.
Collect spermatozoa and suspend them in the ice-cold Hank’s buffer.
Note: Zebrafish spermatozoa are inactive in Hank’s buffer.
>Observation of spermatozoa whose heads are attached to the coverslip (Figure 1)
Attach two fragments of double-sided tape on the 24x60mm coverslip.
Drop 1 µl of spermatozoa/Hank’s buffer between the two fragments of double-sided tape.
Cover with 18x18mm coverslip. The double-sided tapes work as spacers.
Set the coverslips on the inverted microscope and adjust the focus using x40 objective lens.
To activate the spermatozoa, inject 5-10 µl of x1/5 Hank’s buffer to the space between 24x60mm and 18x18mm coverslips.
Note: Injecting excess amount of x1/5 Hank’s buffer may wash out the spermatozoa whose heads were attached to the coverslip. Just a contact of the spermatozoa droplet and the injected x1/5 Hank’s buffer is enough to activate the spermatozoa.
Film the motility of sperm flagella using high-speed camera (1000 fps, bright-field condition).
Note: Motility of zebrafish spermatozoa must be observed immediately after the activation, since the spermatozoa are no longer active after ~1 minutes.
>Observation of free swimming spermatozoa for CASA (Figure 2)
To prevent spermatozoa from attaching to the coverslip, add 2 mg/ml BSA (final concentration) to the spermatozoa/Hank’s buffer and the x1/5 Hank’s buffer.
Attach two fragments of double-sided tape on the 24x60mm coverslip.
Drop 10 µl of x1/5 Hank’s buffer with BSA between the two fragments of double-sided tape.
Add 1 µl of spermatozoa/Hank’s buffer with BSA to the droplet and stir the solution briefly by the pipet tip.
Cover with 18x18mm coverslip and quickly set the sample on the inverted microscope.
Quickly adjust the focus using x20 objective lens and film the motility of spermatozoa using high-speed camera (200 fps, bright-field condition).
Note: The procedure described in “Observation of spermatozoa whose heads are attached to the coverslip” section is not suitable for CASA, since the concentration of spermatozoa is not homogenized.
Note: 24x60mm coverslips, instead of slide glasses, were used because we used an inverted microscope.
Data analysis
>Sperm flagella beating frequency analysis
Open the movie file as a stack image with ImageJ.
Re-slice the stack image at the cross-section of sperm flagella to generate a kymograph.
Measure the beating frequency of sperm flagella from the kymograph.
>Sperm flagella waveform analysis
Open the movie file as a stack image with ImageJ.
Extract images in one beating cycle of sperm flagella.
Trace the sperm flagellum on each image with Adobe Illustrator.
Convert the traced line images to plot values using ImageJ (Analyze Line Graph), and calculate the shear angles.
>CASA
CASA modified for zebrafish was performed as previously reported (Wilson-Leedy and Ingermann, 2007).
Used parameters are shown in Figure 3.
Recipes
>Hank’s buffer
137 mM NaCl
5.4 mM KCl
0.25 mM Na2HPO4
0.44 mM KH2PO4
1.3 mM CaCl2
1.0 mM MgSO4
4.2 mM NaHCO3
References
Knowles, M.R., L.A. Daniels, S.D. Davis, M.A. Zariwala, and M.W. Leigh. 2013. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am. J. Respir. Crit. Care Med. 188:913–22.
doi:10.1164/rccm.201301-0059CI.
Wilson-Leedy, J.G., and R.L. Ingermann. 2007. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology. 67:661–72.
doi:10.1016/j.theriogenology.2006.10.003.
Figures
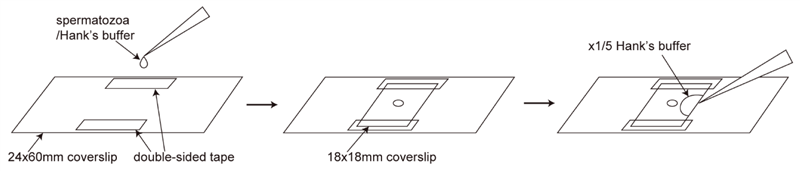
Figure 1. Procedure of the observation of spermatozoa whose heads are attached to the coverslip
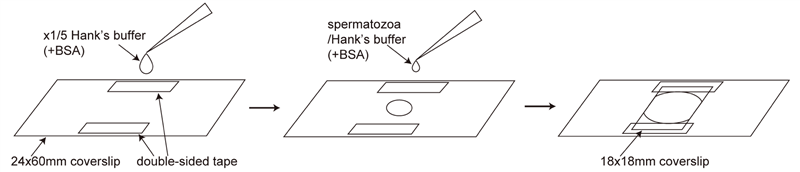
Figure 2. Procedure of the observation of free swimming spermatozoa for CASA
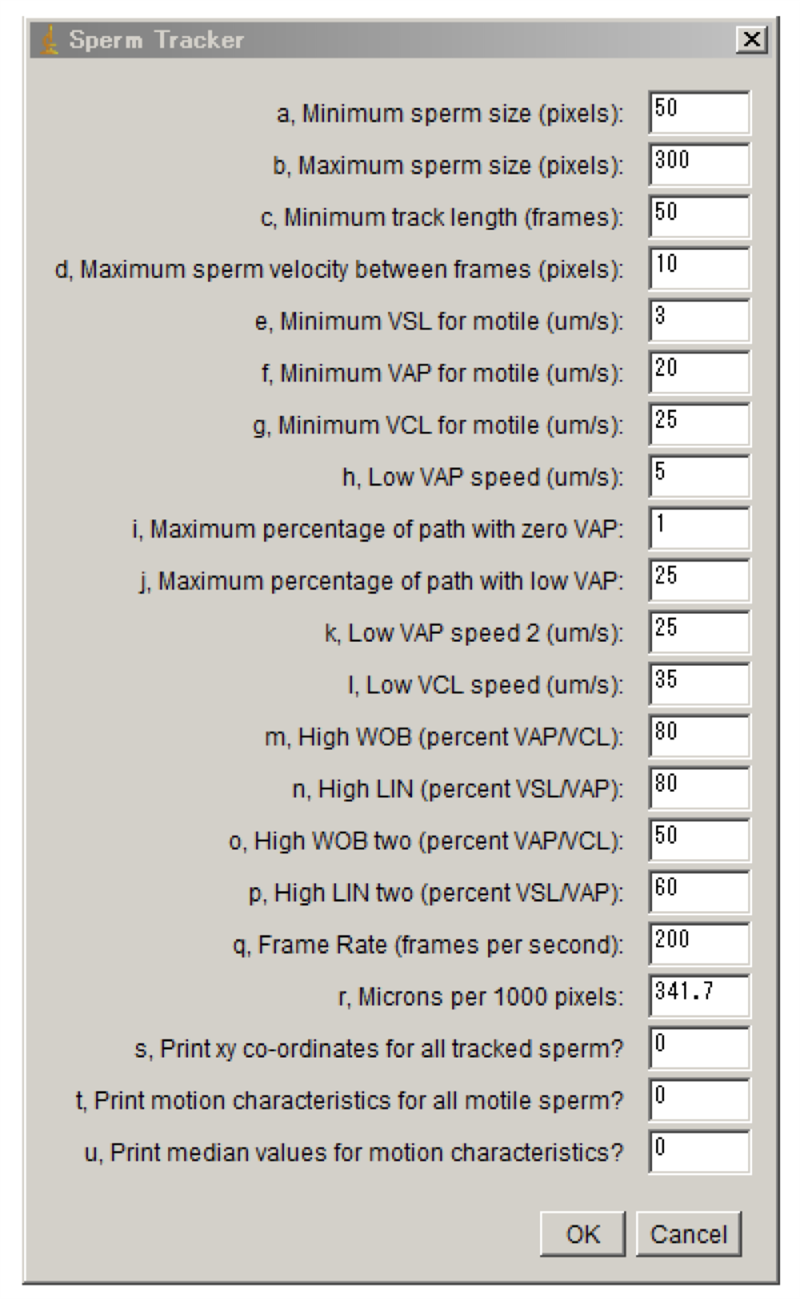
Figure 3. Parameters for CASA
- Kikkawa, M and Yamaguchi, H(2019). Observation and motility assay of zebrafish spermatozoa. Bio-protocol Preprint. bio-protocol.org/prep21.
- Yamaguchi, H., Oda, T., Kikkawa, M. and Takeda, H.(2018). Systematic studies of all PIH proteins in zebrafish reveal their distinct roles in axonemal dynein assembly. eLife. DOI: 10.7554/eLife.36979
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
