Advanced Search
Measurement of mitochondrial iron in a murine heart and cultured cardiomyocytes
Last updated date: Dec 1, 2022 Views: 1063 Forks: 0
Background
Ferroptosis is a form of iron-dependent regulated cell death. It is initiated by oxidative perturbations of the intracellular microenvironment that is under constitutive control by glutathione peroxidase 4 (GPX4) and can be inhibited by iron chelators and lipophilic antioxidants (Galluzzi et al., 2018). We recently demonstrated that doxorubicin, an anthracycline chemotherapeutic agent, causes ferroptosis as a cardiotoxicity, triggered by iron overload in the mitochondria (Tadokoro et al., 2020), and further revealed its mechanism in the heart and cultured cardiomyocytes (Abe et al., 2022). Ferroptosis research is expanding (Stockwell, 2022), and sharing detailed protocols for iron measurement will be critical for scientific advancement. Here, we used the ferrozine method and Mito-FerroGreen to measure iron levels in the mitochondria of a murine heart and culture cardiomyocytes isolated from neonatal rats, respectively.
Animal Ethics
All procedures involving animals and animal care protocols (A20-153, A20-320, A20-329, and A22-009) were approved by the Committee on Ethics of Animal Experiments at the Kyushu University Faculty of Medical and Pharmaceutical Sciences and were performed in accordance with the Guidelines for Animal Experiments of Kyushu University and the Guide for the Care and Use of Laboratory Animals (National Academics).
Measurement of mitochondrial iron in a murine heart
Isolation of mitochondria from murine heart tissues
- The mice were euthanized with an overdose of a mixture containing medetomidine, midazolam, and butorphanol and the hearts were harvested and frozen in liquid nitrogen rapidly.
- Left ventricular tissues (~30 mg) were homogenized in HES buffer (10 mM HEPES, 0.25 M Sucrose, and 1 mM EDTA-2Na) using a disposable Biomasher (BioMasher II).
- The homogenates were centrifuged at 3,000 rpm (800 ×g) for 10 min at 4 °C and the supernatants were collected.
- The supernatants were then centrifuged at 10,000 rpm (9,100 ×g) for 10 min at 4 °C.
- The centrifuged supernatants were then removed and the pellets were suspended with HES buffer.
- The samples were centrifuged at 10,000 rpm (9,100 ×g) for 10 min at 4 °C.
- The procedure described in steps 5–6 was repeated.
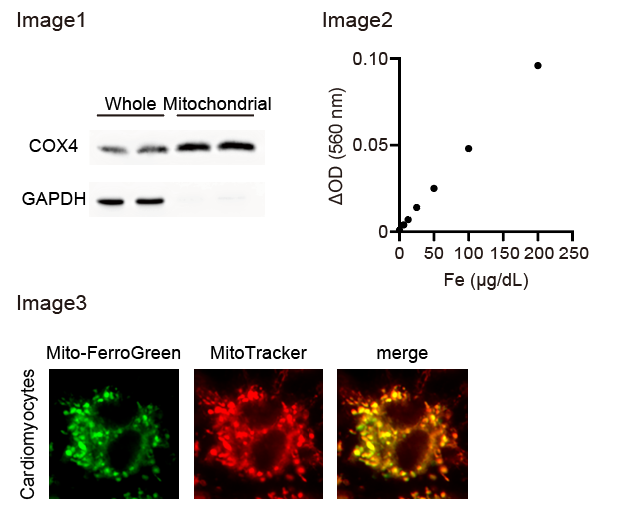
- The supernatants were removed and the pellets (mainly comprising mitochondria; Image 1), were lysed with radioimmunoprecipitation (RIPA) buffer (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P 40 Substitute, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate).
Measurement of iron in mitochondrial lysates
- Mitochondrial lysates were acidified to pH 2 using HCl and centrifuged at 1,000 rpm (100 ×g) for 10 min.
- The supernatants were mixed with R-A buffer provided in Metallo Assay Kit Iron LS, and the absorbance of the samples at 560 nm (determined as OD1) was measured using a microplate reader.
- R-R Chelate Color was then added to the samples, and the absorbance at 560 nm (determined as OD2) was measured 5 min after addition.
- The iron levels of the samples were calculated from the increase in absorbance (ΔOD; OD2-OD1) using the calibration curve (Image 2).
Measurements of mitochondrial iron in living cultured cardiomyocytes
Probing mitochondrial iron using Mito-FerroGreen
- Isolated cultured cardiomyocytes seeded at 2.5×105/mL in collagen I-coated glass base dishes (φ27 mm) were washed three times with phosphate-buffered saline (PBS).
- The cells were incubated with Dulbecco’s Modified Eagle Medium (DMEM) containing 1% penicillin/streptomycin and 5 μM Mito-FerroGreen, a fluorescent probe for mitochondrial ferrous iron (Hirayama et al., 2018), for 30 min at 37 °C in the dark.
- The cells were washed three times with PBS, and the dish containing the cells was finally filled with PBS.
Acquisition of images
- The dish was placed under a fluorescence microscope (Keyence, BZ-X800).
- Fluorescence GFP filter was used to obtain images of mitochondrial ferrous iron. Green fluorescence (Mito-FerroGreen) was merged with red fluorescence (MitoTracker) through the TRITC filter (Image3).
Quantification
- The images obtained from the fluorescence microscope were analyzed by ImageJ.
- Each cardiomyocyte was traced, and its signal intensity (green) was measured as the mitochondrial ferrous iron level of cardiomyocytes.
Materials
- Medetomidine (Meiji Animal Health, VETLI5-A)
- Midazolam (SANDOZ, 614243022)
- Butorphanol (KYOWA Pharmaceutical Industry, DOL04-AS2101)
- HEPES (DOJINDO, 346-01373)
- Sucrose (Wako, 196-00015)
- EDTA-2Na (DOJINDO, 345-01865)
- BioMasher II (nippi, 320102)
- Centrifuge (TOMY SEIKO, MX-300)
- Tris (nacalai tesque, 35406-75)
- HCl (nacalai tesque, 37313-25)
- NaCl (nacalai tesque, 31320-05)
- Nonidet P 40 Substitute (Sigma, 74385-1L)
- Sodium deoxycholate (Merck, 264101-25GM)
- Sodium dodecyl sulfate (NIPPON GENE, 313-90275)
- Metallo Assay Kit Iron LS (Metallogenics, FE31M)
- Microplate reader (Thermo Fisher Scientific, Varioskan LUX)
- Collagen I-coated glass base dish (φ27mm; IWAKI, 4970-011)
- PBS (nacalai tesque, 14249-95)
- DMEM (Sigma-Aldrich, D5796)
- Penicillin/streptomycin (nacalai tesque, 26253-84)
- Mito-FerroGreen (DOJINDO, M489)
- Fluorescence microscope (Keyence, BZ-X800)
- GFP filter (Keyence, OP-87763)
- MitoTracker Red CMXRos (Thermo Fisher Scientific, M7512)
- TRITC filter (Keyence, OP-87764)
- Image J (National Institutes of Health, free software)
Images
Reference
- Abe, K., Ikeda, M., Ide, T., Tadokoro, T., Miyamoto, H.D., Furusawa, S., Tsutsui, Y., Miyake, R., Ishimaru, K., Watanabe, M., et al. (2022). Doxorubicin causes ferroptosis and cardiotoxicity by intercalating into mitochondrial DNA and disrupting Alas1-dependent heme synthesis. Sci Signal 15(758): eabn8017.
- Galluzzi, L., Vitale, I., Aaronson, S.A., Abrams, J.M., Adam, D., Agostinis, P., Alnemri, E.S., Altucci, L., Amelio, I., Andrews, D.W., et al. (2018). Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3): 486-541.
- Hirayama, T., Kadota, S., Niwa, M., and Nagasawa, H. (2018). A mitochondria-targeted fluorescent probe for selective detection of mitochondrial labile Fe(ii). Metallomics 10(6): 794-801.
- Stockwell, B.R. (2022). Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 185(14): 2401-2421.
- Tadokoro, T., Ikeda, M., Ide, T., Deguchi, H., Ikeda, S., Okabe, K., Ishikita, A., Matsushima, S., Koumura, T., Yamada, K.I., et al. (2020). Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 5(9): e132747.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP21K16090 (MI).
- Abe, K, Ikeda, M, Ide, T and Tsutsui, H(2022). Measurement of mitochondrial iron in a murine heart and cultured cardiomyocytes. Bio-protocol Preprint. bio-protocol.org/prep2071.
- Abe, K., Ikeda, M., Ide, T., Tadokoro, T., Miyamoto, H. D., Furusawa, S., Tsutsui, Y., Miyake, R., Ishimaru, K., Watanabe, M., Matsushima, S., Koumura, T., Yamada, K., Imai, H. and Tsutsui, H.(2022). Doxorubicin causes ferroptosis and cardiotoxicity by intercalating into mitochondrial DNA and disrupting Alas1-dependent heme synthesis. Science Signaling 15(758). DOI: 10.1126/scisignal.abn8017
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
