Advanced Search
Chromatin and whole cell Flow cytometry
Last updated date: Jan 14, 2020 Views: 1327 Forks: 0
Chromatin Fractionation for Flow Cytometry
Note: This protocol was optimized for use with anti MCM2 BM28 antibody (BD biosciences, 610700, mouse monocolonal). antiMCM3 (Bethyl A300-192A rabbit) and yH2AX CST 9718S. Other proteins/antibodies may require different extraction/labeling conditions, see papers below among others:
Forment Josep V, Jackson Stephen P. A flow cytometry–based method to simplify the analysis and quantification of protein association to chromatin in mammalian cells. Nature Protocols. 2015;10(9):1297-1307.
Håland TW, Boye E, Stokke T, Grallert B, Syljuåsen RG. Simultaneous measurement of passage through the restriction point and MCM loading in single cells. Nucleic Acids Research. 2015;(C):gkv744.
Matson et al, 2017. “Rapid DNA replication origin licensing protects stem cell pluripotency” eLife. https://elifesciences.org/articles/30473
Note in original eLife paper Donkey anti Mouse488 cat# should be 715-545-150.
Carrol et al 2018. Lgr5+ intestinal stem cells reside in an unlicensed G1 phase
http://jcb.rupress.org/content/217/5/1667
Matson, J. P., House, A. M., Grant, G. D., Wu, H., Perez, J., and Cook, J. G. (2019) Intrinsic checkpoint deficiency during cell cycle re-entry from quiescence. Journal of Cell Biology. 218(7): 2169-2184. Link: https://doi.org/10.1083/jcb.201902143
Cell Plating:
For chromatin flow a ~60-80% confluent 6cm per sample is sufficient (plate ~300K cells day before harvest depending on the cell line, about 500-600k at collection).
Remember control samples: Single color controls. All stains sample for setting voltages/gates. Negative antibody/negative EDU+DAPI to gate positives. Separate controls are need if whole cells and chromatin are being run at the same time, and voltages will need to be adjusted differently for whole cells and chromatin.
If EdU labeling is desired, add EdU to cells before harvesting, refer to the EdU labeling protocol before antibody staining. For whole cell fixing, follow EdU protocol, then return to this protocol for antibody staining
Chromatin Extraction and Fixation:
Keep cells cold/on ice while extracting.
1. Trypsinize cells into a single cell suspension and stop trypsinization by adding media with FBS. Pipet up and down well to break up and cell clumps.
Good trypsinization and mixing is crucial to prevent cell clumping.
Collect into 1.7mL eppendorfs if possible
2. Centrifuge 2000g, 3 minutes
3. Aspirate gently, resuspend with 500uL cold PBS, centrifuge 2000g, 3min
3b. For whole cell staining (CDT1 etc), skip to fixation, step 7 below. Then permeabilize in 1 mL 0.5% triton-100 in PBS for 15 min RT, mix well by pipette, then proceed onward to the storage step 9-10
4. Aspirate gently, resuspend thoroughly with 500uL cold CSK +0.5% Triton X-100 + protease/phosphatase inhibitors. Incubate on ice for 5-10 minutes.
For detection of associated vs loaded proteins use CSK with 300mM NaCl +0.5% Triton X-100 + inhibitors instead of normal 100mM NaCl CSK. Incubate on ice, proceed with protocol as normal.
5. Add 1mL 1% BSA + PBS directly to sample and gently pipette to mix (BSA helps cells pellet)
6. Centrifuge 2000g, 3 minutes
7. Aspirate gently, resuspend cells very thoroughly 500uL PBS + 4% paraformaldehyde, incubate at RT for 15 minutes
It is critical to resuspend cells into a single cell suspension without clumps. Any clumping cannot be undone after fixation.
8. Add 1mL 1% BSA + PBS directly to sample and gently pipette to mix.
9. Centrifuge 2000g, 7 minutes. Note the pellet is harder to see/ more diffuse after the fixing step. Can spin twice if you have difficulty seeing it. Be more careful aspirating this step onward, aspirating via pipette is recommended instead of vacuum.
10. Aspirate gently, resuspend with 1mL 1% BSA + PBS.
Store here at 4 °C in 1% BSA, PBS if needed, or proceed directly to EdU or antibody staining. Good for a week or two.
For EdU samples, label EdU before MCM2 , it seems to be better for MCM2 staining.
see EdU labeling protocol below, taken from our standard EdU labeling protocol. If not EdU labeling, skip to antibody staining.
Cell cycle analysis- EdU labeling
- (before collection) Label cells with EdU using a final concentration of 10 uM EdU in media.
- Add dropwise to cells, or by media exchange into 10uM EdU.
- 30 minute of labeling is sufficient. Shorter would probably work, but has not been tested.
After Extraction/Fixation Step 10 above
- Pellet cells. 7 min 2000g.
- Prepare labeling reaction solutions during prior centrifugation.
- Prepare 500 mM ascorbic acid in water.
- Dissolve 0.88g ascorbic acid per 10 ml of water, vortex into solution.
- Prepare fresh each time and throw away after use.
- Prepare labeling reaction solution master mix. For each reaction, combine:
- PBS: 400 ul
- CuSO4 (100 mM stock): 5 uL(1 mM final conc.)
- Alexa Fluor 647 Azide (or 488 or any other fluorophore) (1 mM stock): ~0.5 uL/rxn (final concentration of 0.2 – 2 uM)
- this may be different for different cell types and fluorescent azides
- Ascorbic acid (500 mM stock): 100 uL (100 mM final conc.).
- Add ascorbic acid last, immediately before use
- Vortex labeling mix well before use
- Resuspend cell pellet in 500 uL labeling reaction solution.
- Incubate at room temperature for 30 minutes, protected from light (in drawer or wrapped in foil).
- Add 1ml of PBS + 1% BSA + 0.1% NP-40.
Antibody Staining
If cells are not already in 1.7mL Eppendorf tubes, transfer now.
1. Centrifuge cells, 2000g, 7 minutes
2. Aspirate gently. Remove as much liquid as possible, down to ~5uL or less (use a pipette manually). Don't suck up your cells!
The lowest volume possible is critical for equal staining between samples but don’t suck up your cells!
3. Resuspend cells with 50uL of 1% BSA + PBS + 0.1% NP-40, with primary antibody anti MCM2 BM28 diluted 1:200 (BD Biosciences, 610700 mouse monocolonal) for antiMCM3 (Bethyl A300-192A rabbit use 1:200) RPA2 ab2175 mouse use 1:200 and yH2AX Rb CST 9718S use 1:200. For whole cell CDT1 ab202067 Rb use 1:100.
For controls without any primary, just add the same solution without primary antibody.
4. Incubate at 37 °C for 1 hour in dark (wrapped in foil is fine).
5. Add 1mL 1% BSA + PBS + 0.1% NP-40 and gently pipette up and down to mix
6. Centrifuge 2000g 7min.
7. Aspirate gently. Remove as much liquid as possible, down to ~5uL or less (use a pipette manually if needed). Don't suck up your cells!!!
The lowest volume possible is critical for equal staining between samples
8. Resuspend cells with 200uL of 1% BSA + PBS + 0.1% NP-40, with secondary antibody 1:1000 (DantiM-488 for BM28, or other fluorophores as desired)
9. Incubate at 37 °C for 1 hour. IN THE DARK!!!
10. Add 1mL 1% BSA + PBS + 0.1% NP-40 and gently pipette to mix
11. Centrifuge 2000g 7min
12. Aspirate gently. Resuspend cells in 500uL 1% BSA + PBS + 0.1% NP-40, 1 ug/mL DAPI and 100 ug/mL RNAase. Leave in fridge overnight (IN THE DARK!!!) and run on flow cytometer next day (or incubate at 37°C for 1hour then run same day, results in essentially identical dapi staining)
For proper gating, see Matson, 2017, eLife Fig 1-S1 and the Nature Protocols paper, but use DAPI for doublets as we normally do. Examples are also below
Updated 03-06-2018 JM
CSK: (identical to CSK for westerns)
10 mM Pipes pH 7.0 (0.36 g/ 100 ml)
300 mM sucrose (10.27 g/ 100 ml)
100 mM NaCl (0.58 g/ 100 ml) (use 300mM NaCl for loaded vs associated)
3 mM MgCl2 (0.061 g/ 100 ml or 0.3 ml of 1M stock)
Add protease/phosphatase inhibitors right before experiment
I make CSK fresh once a week and store in the fridge without detergents. 1% BSA in PBS should also be relatively fresh, no more than a week.
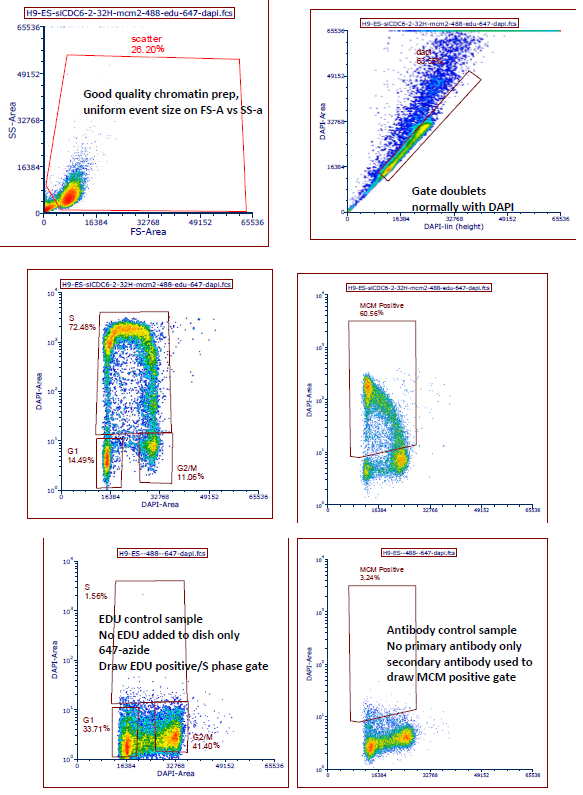
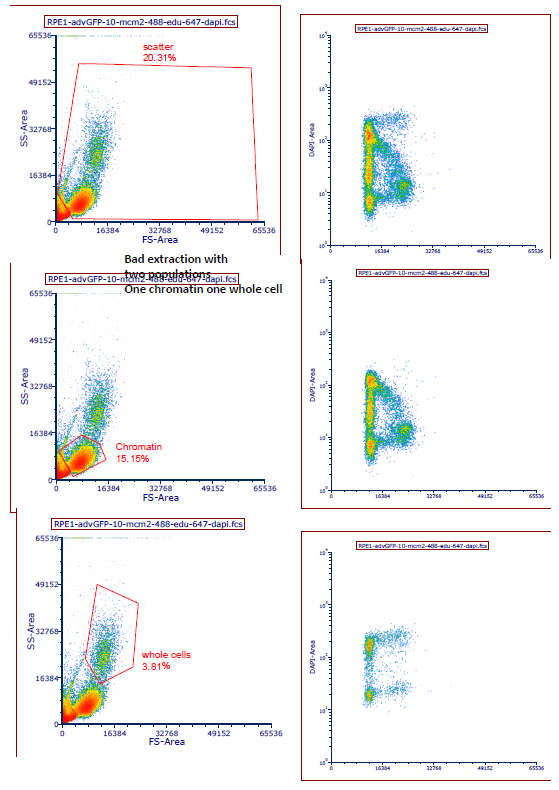
- Matson, J and Cook, J(2020). Chromatin and whole cell Flow cytometry. Bio-protocol Preprint. bio-protocol.org/prep199.
- Matson, J. P., Dumitru, R., Coryell, P., Baxley, R. M., Chen, W., Twaroski, K., Webber, B. R., Tolar, J., Bielinsky, A., Purvis, J. E. and Cook, J. G.(2017). Rapid DNA replication origin licensing protects stem cell pluripotency. eLife. DOI: 10.7554/eLife.30473
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
